Question: Using the table of SI unit prefixes in your notes (Chap 2, page 9), complete the following conversion factors. Answers should be written in decimal
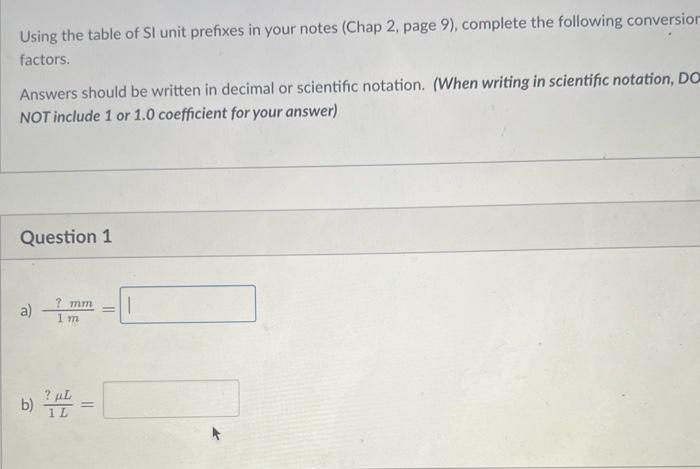
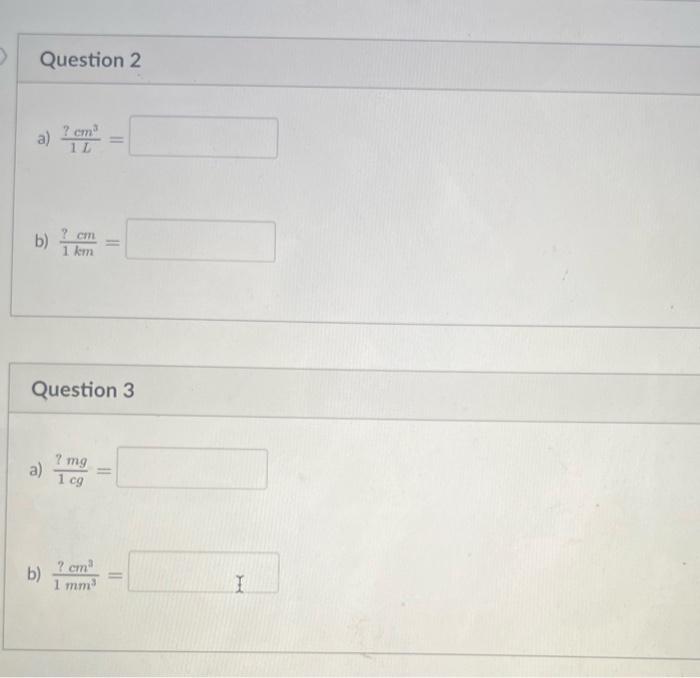
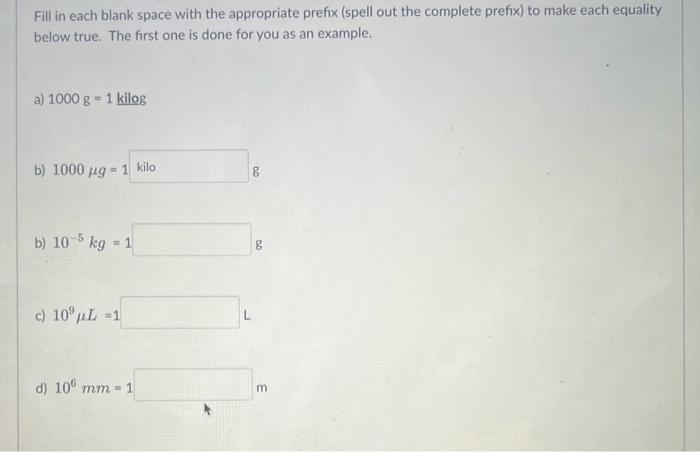
Using the table of SI unit prefixes in your notes (Chap 2, page 9), complete the following conversion factors. Answers should be written in decimal or scientific notation. (When writing in scientific notation, DO NOT include 1 or 1.0 coefficient for your answer) Question 1 a) 1m?mm= b) 1L?L= 1L?cm3= 1km?cm= uestion 1cg?mg= 1mm?cm3= Fill in each blank space with the appropriate prefix (spell out the complete prefix) to make each equality below true. The first one is done for you as an example. a) 1000g=1 kilog b) 1000g= : g b) 105kg=1 g c) 109L=1 d) 106mm=1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


