Question: Using the table showing partial pressures for the formation of ammonia and corresponding equilibrium constants, show that at 101 bar, apparent equilibrium constants equals 0.0072
Using the table showing partial pressures for the formation of ammonia and corresponding equilibrium constants, show that at 101 bar, apparent equilibrium constants equals 0.0072 and the thermodynamic equilibrium constant equals 0.0064.
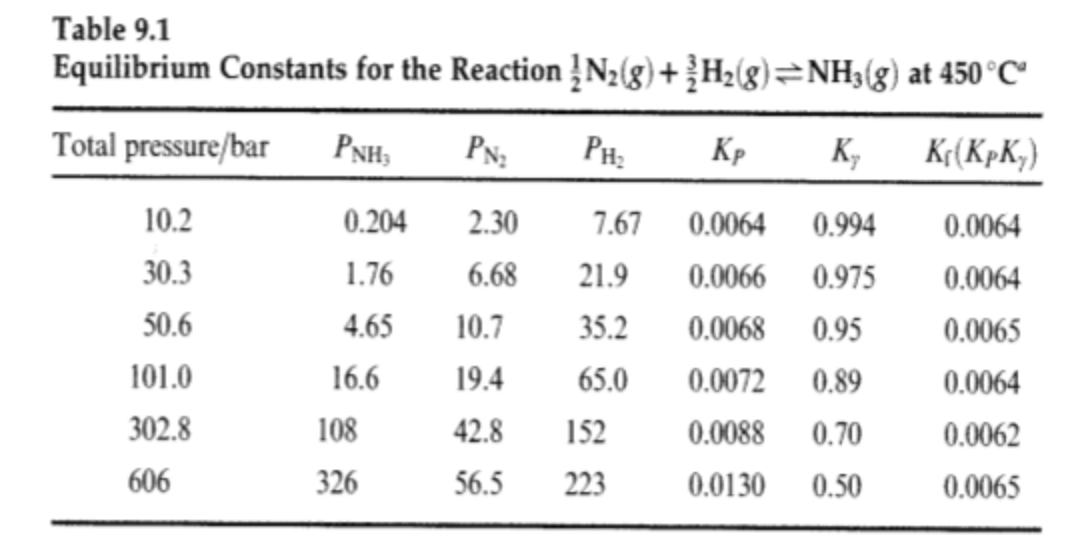
Table 9.1 Equilibrium Constants for the Reaction N2(g) + H2(8)=NH3(g) at 450C Total pressure/bar PNH PN , K, K(KPK) 2.30 7.67 0.0064 0.0064 0.204 1.76 6.68 21.9 0.0066 10.2 30.3 50.6 101.0 302.8 10.7 4.65 16.6 108 326 35.2 65.0 152 223 19.4 0.994 0.975 0.95 0.89 0.70 0.50 0.0068 0.0072 0.0088 0.0130 0.0064 0.0065 0.0064 0.0062 42.8 606 56.5 0.0065
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


