Question: Using the unknown raw data in question 2 ( the coloured circles represent if there a precipitate, Y means yes and N means No), identify
Using the unknown raw data in question 2 ( the coloured circles represent if there a precipitate, Y means yes and N means No), identify which compounds belong to which unknowns and identify the precipitates. Please read the instructions 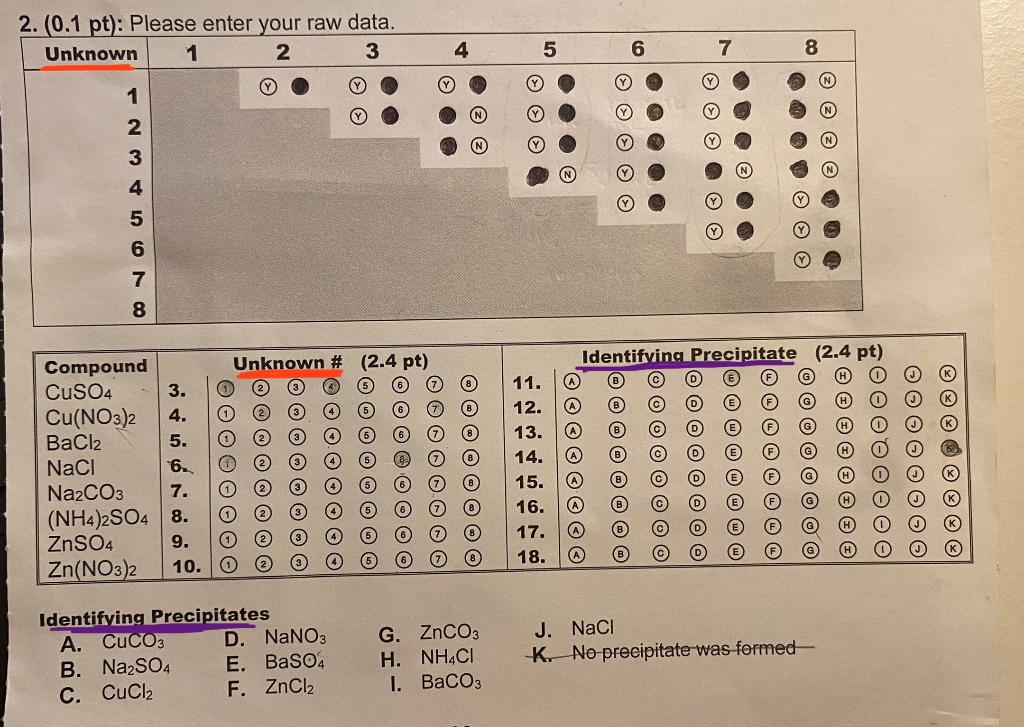

1 Raw Data Set: INSTRUCTIONS!!!! Indicate which unknown combinations created a precipitate by filling in the appropriate bubble in the table provided. Fill in if a precipitate formed or if a precipitate did not form. Unknown Identification and Identifying Precipitates Identify unknown by bubbling in the correct unknown \#. Identify which precipitate was formed by bubbling in the correct letter from the "Identifying Precipitates" list. There may be more than one correct answer. Please choose ONLY ONE correct answer. Example: Unknown Identification and Identifying Precipitates A student determines from their raw data that Unknown \# 2 is CuSO4 and bubbled in " 2 " for the CuSO4 unknown \# entry in the data table. A student determined that there are two possible precipitates that could form by combining a CuSO4 solution with the remaining 7 solutions (their equations are written below). CuSO4(aq)+Na2CO3(aq)CuCO3(s)+Na2SO4(aq)CuSO4(aq)+BaCl2(aq)BaSO4(s)+CuCl2(aq) In this case, there is more than one correct answer. The instructions state to choose ONLY ONE correct answer. In this example, the student has chosen BaSO4 as their identifying precipitate and bubbled in "E" for the CuSO4 identifying precipitate entry in the data table. (NOTE: If the student had chosen CuCO3 as the identifying precipitate and bubbled in " A ", this would also be a correct solution.) 1. Some metal ions give solutions with a characteristic colour. R a) Most Cu2+ ions are blue ) Most Zn2+ ions are colourless You are correct. Your receipt no. is 162-6027 1 Raw Data Set: INSTRUCTIONS!!!! Indicate which unknown combinations created a precipitate by filling in the appropriate bubble in the table provided. Fill in if a precipitate formed or if a precipitate did not form. Unknown Identification and Identifying Precipitates Identify unknown by bubbling in the correct unknown \#. Identify which precipitate was formed by bubbling in the correct letter from the "Identifying Precipitates" list. There may be more than one correct answer. Please choose ONLY ONE correct answer. Example: Unknown Identification and Identifying Precipitates A student determines from their raw data that Unknown \# 2 is CuSO4 and bubbled in " 2 " for the CuSO4 unknown \# entry in the data table. A student determined that there are two possible precipitates that could form by combining a CuSO4 solution with the remaining 7 solutions (their equations are written below). CuSO4(aq)+Na2CO3(aq)CuCO3(s)+Na2SO4(aq)CuSO4(aq)+BaCl2(aq)BaSO4(s)+CuCl2(aq) In this case, there is more than one correct answer. The instructions state to choose ONLY ONE correct answer. In this example, the student has chosen BaSO4 as their identifying precipitate and bubbled in "E" for the CuSO4 identifying precipitate entry in the data table. (NOTE: If the student had chosen CuCO3 as the identifying precipitate and bubbled in " A ", this would also be a correct solution.) 1. Some metal ions give solutions with a characteristic colour. R a) Most Cu2+ ions are blue ) Most Zn2+ ions are colourless You are correct. Your receipt no. is 162-6027
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


