Question: Using the water-salt phase diagram below, answer the following questions: 10 50 B 40 Liquid (brine) 0 1 30 20 Temperature, C 10 lce Salt
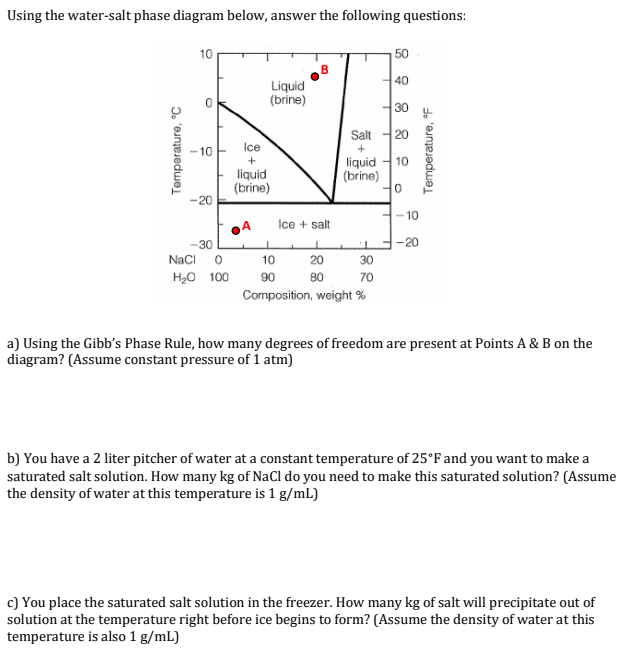
Using the water-salt phase diagram below, answer the following questions: 10 50 B 40 Liquid (brine) 0 1 30 20 Temperature, C 10 lce Salt + liquid (brine) Temperature, F + T 10 liquid (brine) 0 20 -10 A Ice + salt - 30 NaCl 0 H0 100 1 1 10 20 30 90 BO 70 Composition, weight % a) Using the Gibb's Phase Rule, how many degrees of freedom are present at Points A & B on the diagram? (Assume constant pressure of 1 atm) b) You have a 2 liter pitcher of water at a constant temperature of 25F and you want to make a saturated salt solution. How many kg of NaCl do you need to make this saturated solution? (Assume the density of water at this temperature is 1 g/mL) c) You place the saturated salt solution in the freezer. How many kg of salt will precipitate out of solution at the temperature right before ice begins to form? (Assume the density of water at this temperature is also 1 g/mL) Using the water-salt phase diagram below, answer the following questions: 10 50 B 40 Liquid (brine) 0 1 30 20 Temperature, C 10 lce Salt + liquid (brine) Temperature, F + T 10 liquid (brine) 0 20 -10 A Ice + salt - 30 NaCl 0 H0 100 1 1 10 20 30 90 BO 70 Composition, weight % a) Using the Gibb's Phase Rule, how many degrees of freedom are present at Points A & B on the diagram? (Assume constant pressure of 1 atm) b) You have a 2 liter pitcher of water at a constant temperature of 25F and you want to make a saturated salt solution. How many kg of NaCl do you need to make this saturated solution? (Assume the density of water at this temperature is 1 g/mL) c) You place the saturated salt solution in the freezer. How many kg of salt will precipitate out of solution at the temperature right before ice begins to form? (Assume the density of water at this temperature is also 1 g/mL)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


