Question: Using this equation: 4.29 Niobium forms a substitutional solid solution with (4) vanadium. Compute the number of niobium atoms per cubic centimeter for a niobium-vanadium
 Using this equation:
Using this equation: 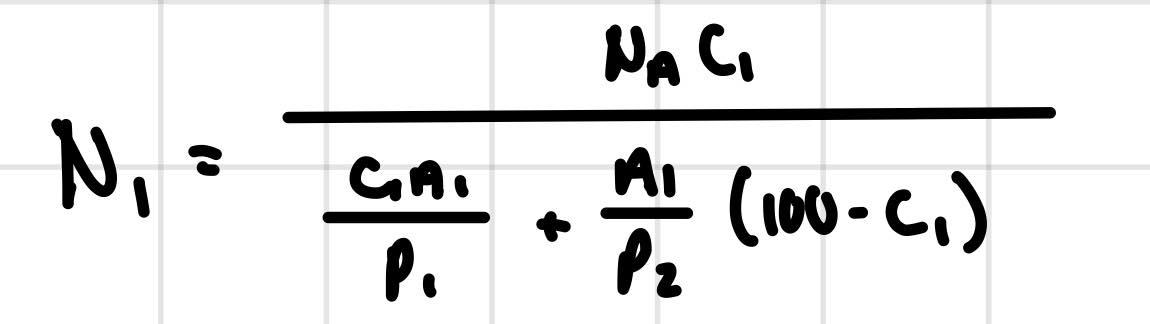
4.29 Niobium forms a substitutional solid solution with (4) vanadium. Compute the number of niobium atoms per cubic centimeter for a niobium-vanadium alloy that contains 24wt%Nb and 76wt%V. The densities of pure niobium and vanadium are 8.57 and 6.10g/cm3, respectively. N1=P1cn1+P2n1(100C1)NnC1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


