Question: Using transition-state theory, set up the pre-exponential factor for the rate constant for the reaction. Cl+H2HCl+H Assume a linear activation complex and assume low T
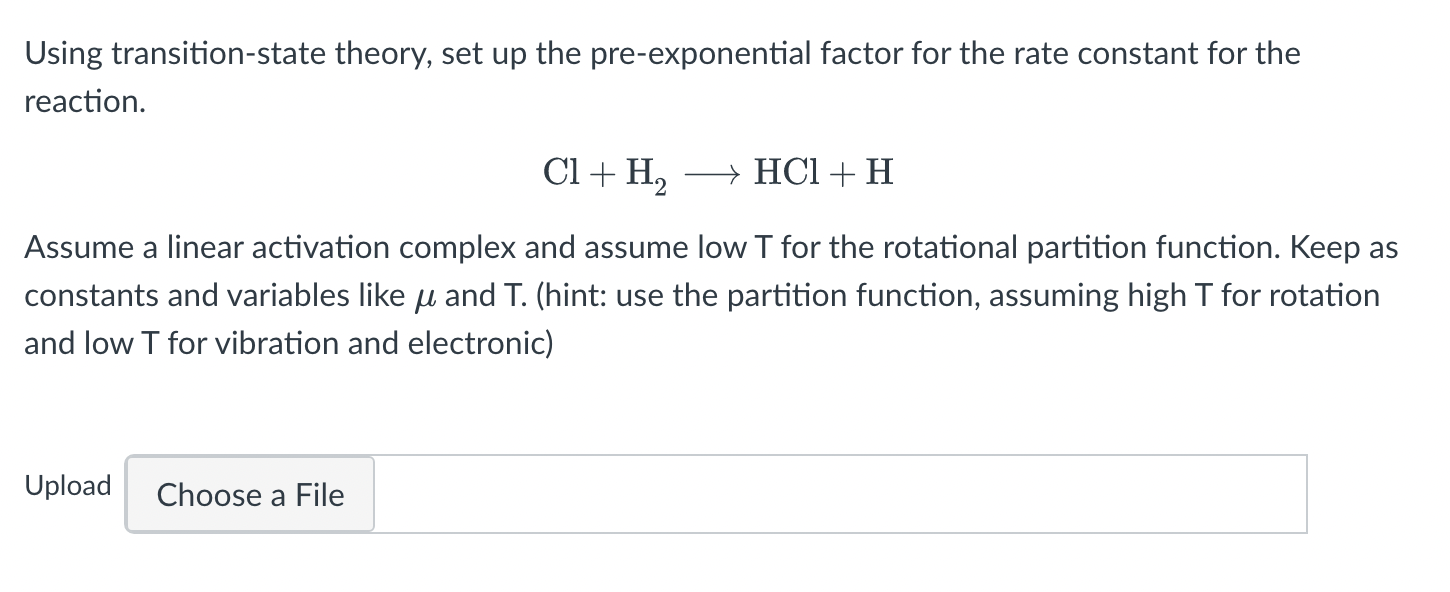
Using transition-state theory, set up the pre-exponential factor for the rate constant for the reaction. Cl+H2HCl+H Assume a linear activation complex and assume low T for the rotational partition function. Keep a constants and variables like and T. (hint: use the partition function, assuming high T for rotation and low T for vibration and electronic)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


