Question: Using values from Appendix C of your textbook, calculate the value of AH for each of the following reactions. (8) 2 SO2(g) + O2(g) -
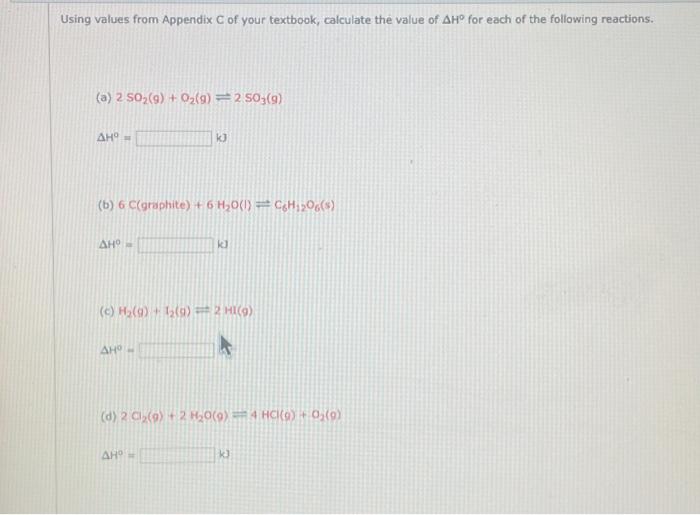
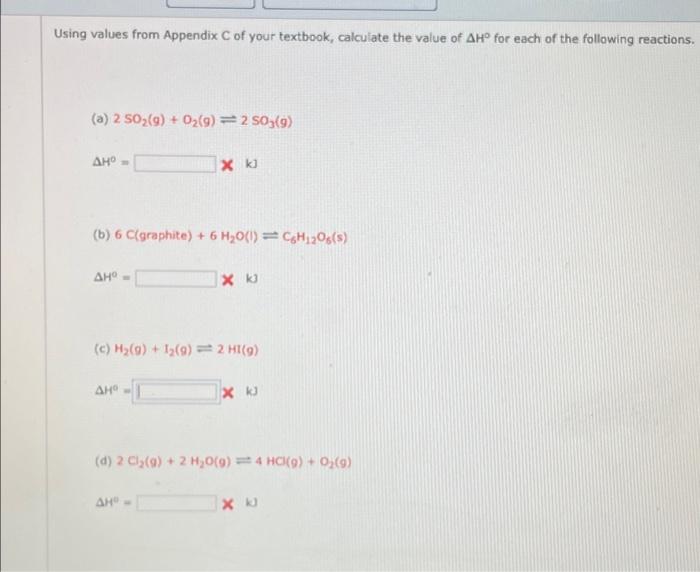
Using values from Appendix C of your textbook, calculate the value of AH for each of the following reactions. (8) 2 SO2(g) + O2(g) - 2 S05(9) (6) 6 C(graphite) + 6 Ho() CHOS) 9 k (6) () 12 ) 2 ) (0) 2 O2() 2 H10 (0) H4 HCl (O) O () Using values from Appendix C of your textbook, calculate the value of AH for each of the following reactions. () 2 50,(9) + O2(9) = 2 s0,09) (b) 6 ctgraphite) +6H2O(1) = CH2005) (c) ) + (9) = 2 HT(9) k (d) 2 Ci (0) + 2H,0(0) 2 4 (0) + 0 (0)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


