Question: Using values from the table of standard reduction potentials, calculate the cell potential in V) of the following cells. (a) Ni(s) | Ni2+ (aq) ||
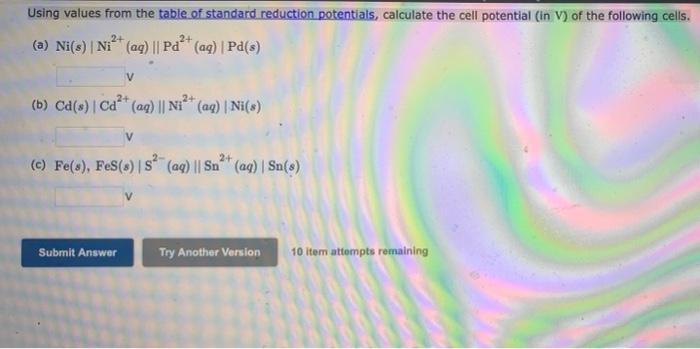
Using values from the table of standard reduction potentials, calculate the cell potential in V) of the following cells. (a) Ni(s) | Ni2+ (aq) || Pd** (ag) | Pd(s) 2+ 24 V (b) Ca(s) Ca+ (aq) || Ni2+ (aq) |Ni(s) 2- 2+ (C) Fe(s), Fe(s) is? (aq) || Sn** (ag) | Sn(s) Submit Answer Try Another Version 10 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


