Question: Utilizing the data from the table directly below, I need help filling out the differential analysis tables for the differing reactant concentrations. I am extremely
Utilizing the data from the table directly below, I need help filling out the differential analysis tables for the differing reactant concentrations. I am extremely confused about how to complete this type of analysis and would appreciate the aid. Thank you in advance!
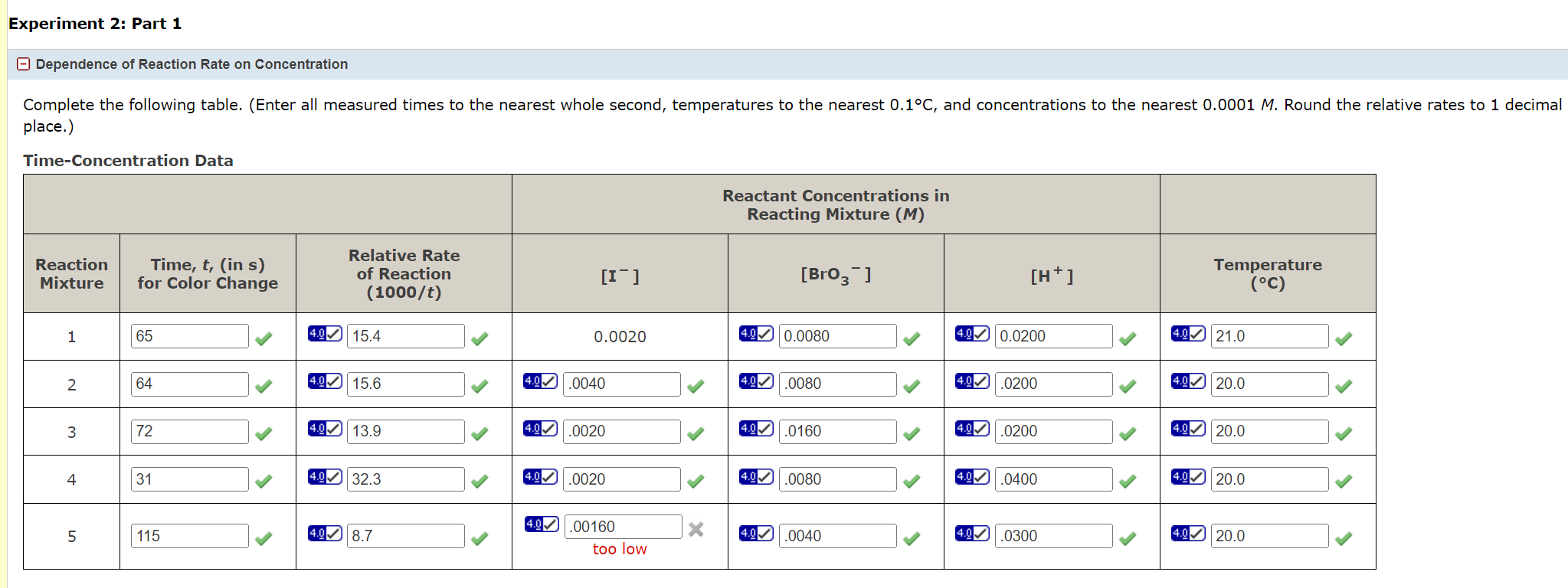
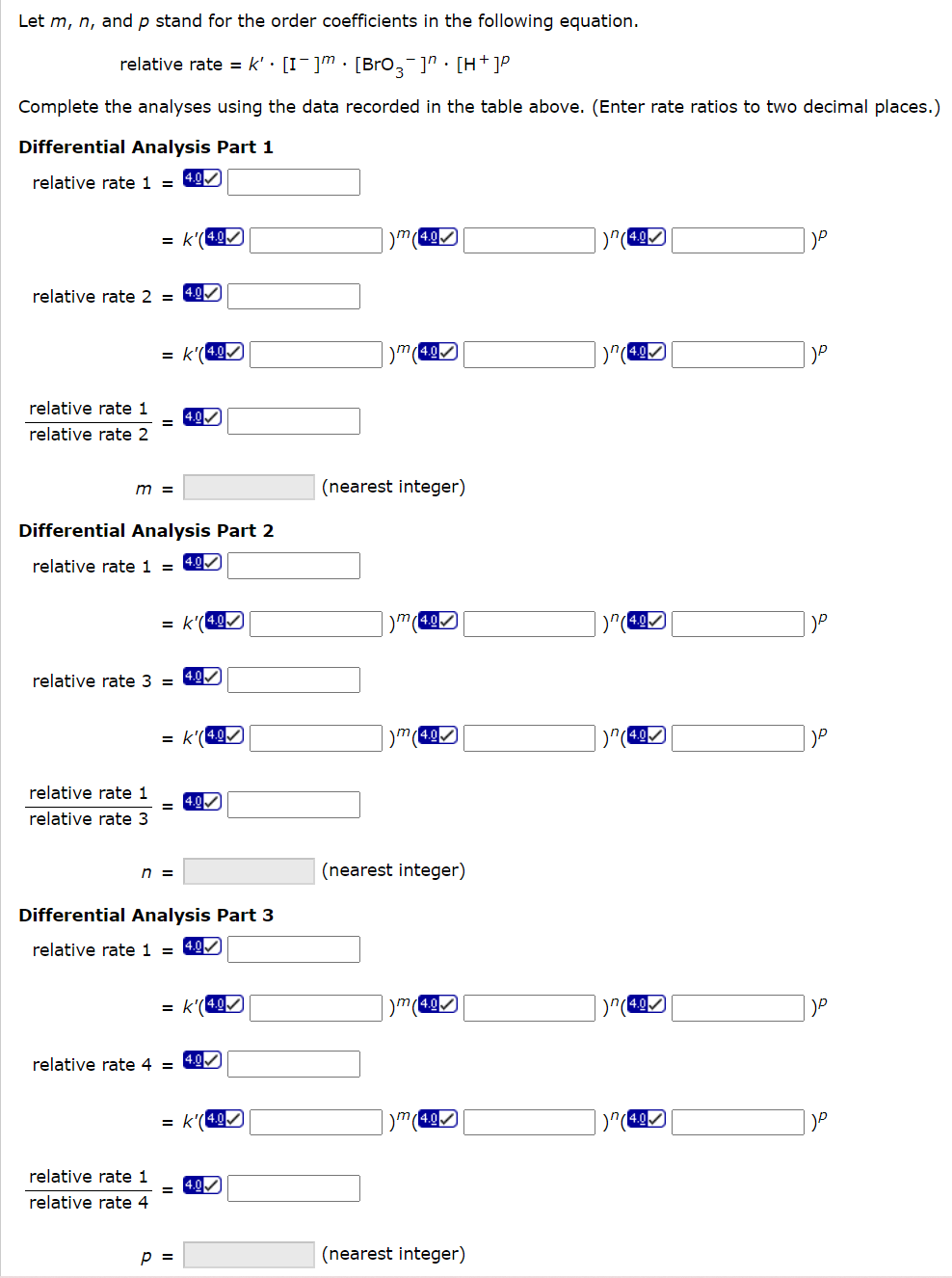
place.) Time-Concentration Data Let m,n, and p stand for the order coefficients in the following equation. relativerate=k[I]m[BrO3]n[H+]p Complete the analyses using the data recorded in the table above. (Enter rate ratios to two decimal p Differential Analysis Part 1 relative rate 1= =k(4.0)m(4.0)n()p relative rate 2= =k(4.9)m(4.0))n()p relativerate2relativerate1= m=(nearestinteger) Differential Analysis Part 2 relative rate 1= =k(4.0)m(4.0)n(4.0)p relative rate 3= =k(4.0)m(1)n()p relativerate3relativerate1= n= (nearest integer) Differential Analysis Part 3 relative rate 1= =k(4.0)m(4.0)n(4.0)p relative rate 4= =k(4.00)m(1)n()p relativerate4relativerate1=4.00 p= (nearest integer) place.) Time-Concentration Data Let m,n, and p stand for the order coefficients in the following equation. relativerate=k[I]m[BrO3]n[H+]p Complete the analyses using the data recorded in the table above. (Enter rate ratios to two decimal p Differential Analysis Part 1 relative rate 1= =k(4.0)m(4.0)n()p relative rate 2= =k(4.9)m(4.0))n()p relativerate2relativerate1= m=(nearestinteger) Differential Analysis Part 2 relative rate 1= =k(4.0)m(4.0)n(4.0)p relative rate 3= =k(4.0)m(1)n()p relativerate3relativerate1= n= (nearest integer) Differential Analysis Part 3 relative rate 1= =k(4.0)m(4.0)n(4.0)p relative rate 4= =k(4.00)m(1)n()p relativerate4relativerate1=4.00 p= (nearest integer)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


