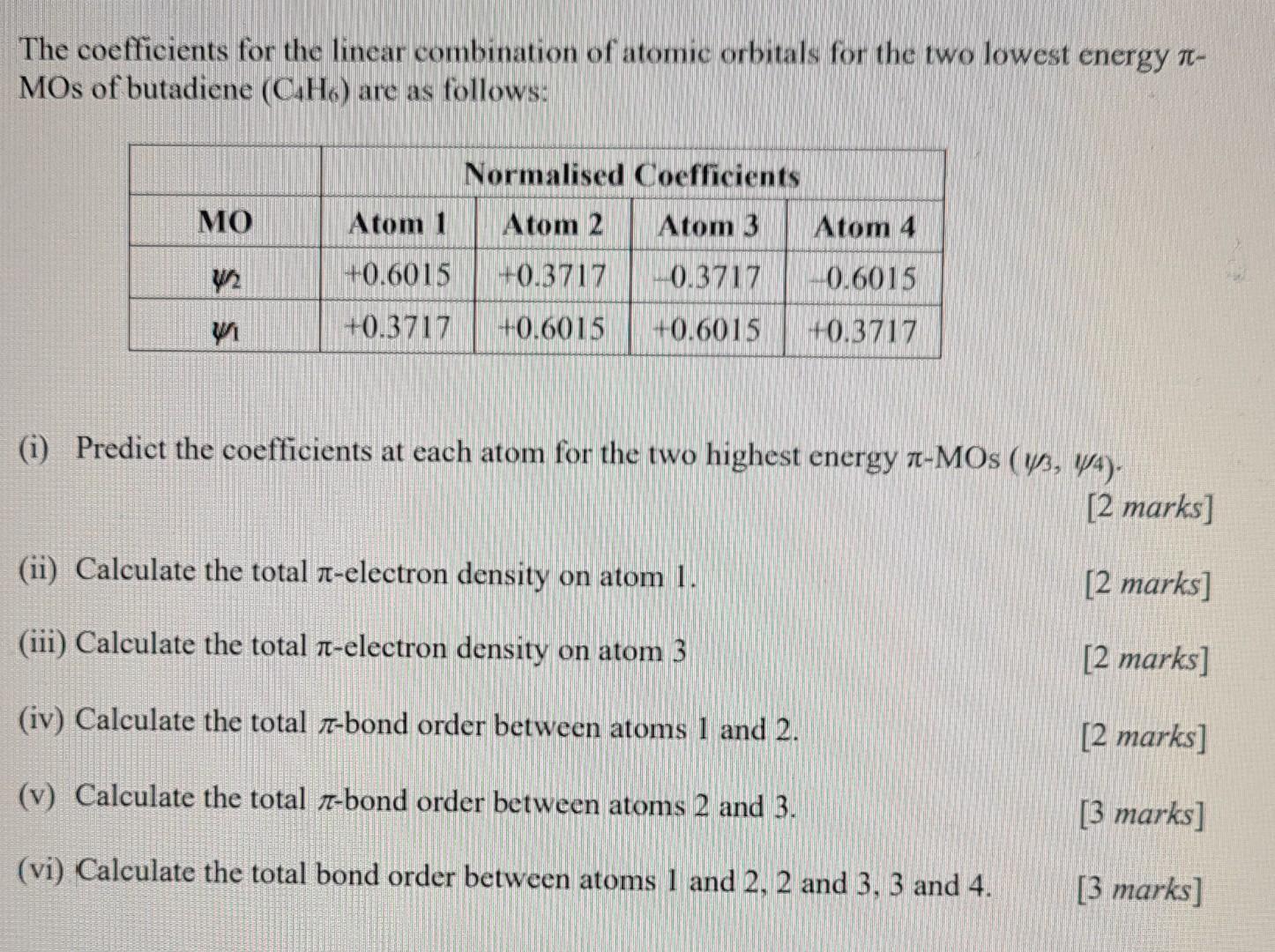
Question: v and vi please The coefficients for the linear combination of atomic orbitals for the two lowest energy MOs of butadiene (CH) are as follows:
v and vi please
The coefficients for the linear combination of atomic orbitals for the two lowest energy MOs of butadiene (CH) are as follows: MO Normalised Coefficients Atom 1 Atom 2 Atom 3 Atom 4 +0.6015 +0.3717 -0.3717 | -0.6015 +0.3717 +0.6015 +0.6015 +0.3717 (1) Predict the coefficients at each atom for the two highest energy r-MOs (Y3, y4). [2 marks] (ii) Calculate the total 7-electron density on atom 1. [2 marks] (iii) Calculate the total 1-electron density on atom 3 [2 marks] (iv) Calculate the total i-bond order between atoms 1 and 2. [2 marks] (v) Calculate the total 7-bond order between atoms 2 and 3. [3 marks] (vi) Calculate the total bond order between atoms 1 and 2, 2 and 3, 3 and 4. [3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


