Question: values allow prediction of redox reactions Example: Given the half reactions below, calculate the free energy change of the spontaneous redox reaction? (F=96.5kJ/mol) NAD++2H++2eNADH+H+-ketoglutarate+CO2+2H++2eisocitrate=0.32V=0.38V
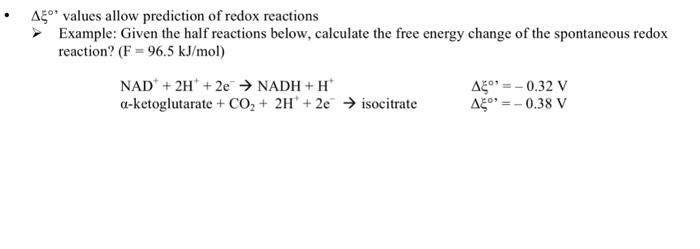
values allow prediction of redox reactions Example: Given the half reactions below, calculate the free energy change of the spontaneous redox reaction? (F=96.5kJ/mol) NAD++2H++2eNADH+H+-ketoglutarate+CO2+2H++2eisocitrate=0.32V=0.38V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


