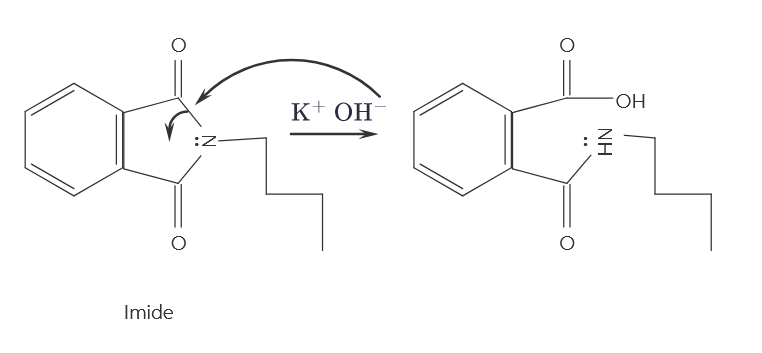
Question: Very simple question: Where does the lone pair on the ring go ? I understand that the hydroxyl group attacks the carbonyl carbon ( s
Very simple question: Where does the lone pair on the ring go
I understand that the hydroxyl group attacks the carbonyl carbonsI am aware that it breaks both bonds to make OH bonds on either end; however, I do not know where the lone pair of electrons that form the bond to nitrogen go Do they just off or do they go to the nitrogen or what? Please help me understand. I thumbs up as soon as I can if the answer helps me
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


