Question: View Policies Show Attempt History Current Attempt in Progress How many atoms of hydrogen are found in 8.49 mol of nonane, C9H20? i e Textbook
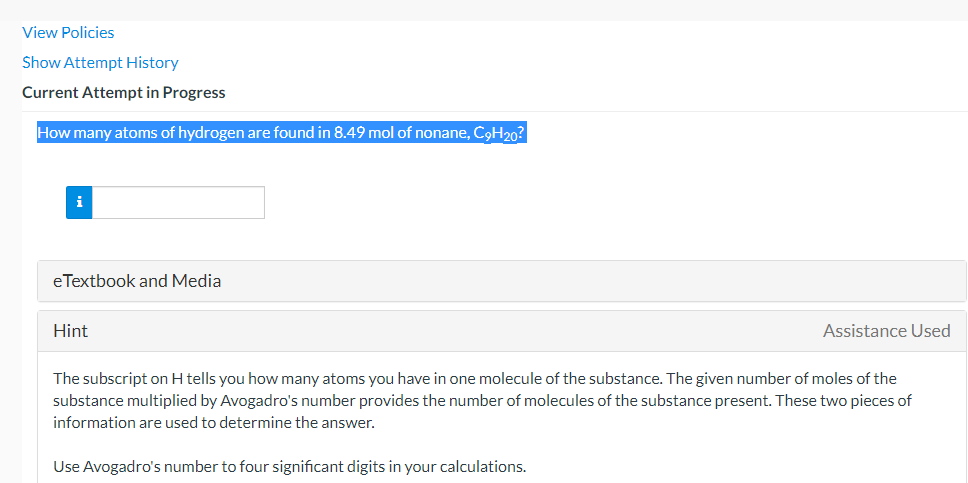
View Policies Show Attempt History Current Attempt in Progress How many atoms of hydrogen are found in 8.49 mol of nonane, C9H20? i e Textbook and Media Hint Assistance Used The subscript on H tells you how many atoms you have in one molecule of the substance. The given number of moles of the substance multiplied by Avogadro's number provides the number of molecules of the substance present. These two pieces of information are used to determine the answer. Use Avogadro's number to four significant digits in your calculations
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


