Question: Voice Editor Determine the Calculated Final Temperature by using the equation learned in the lecture TF mn Th +mcTe (mc+mn) mi= mass of the hot
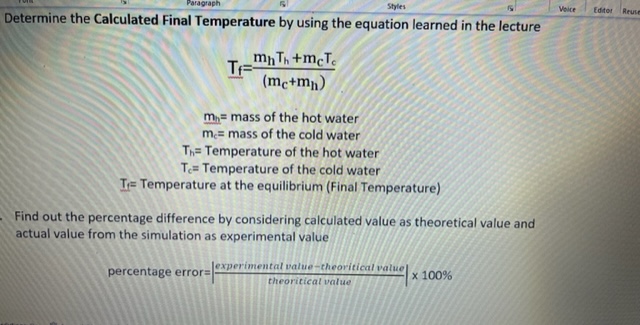
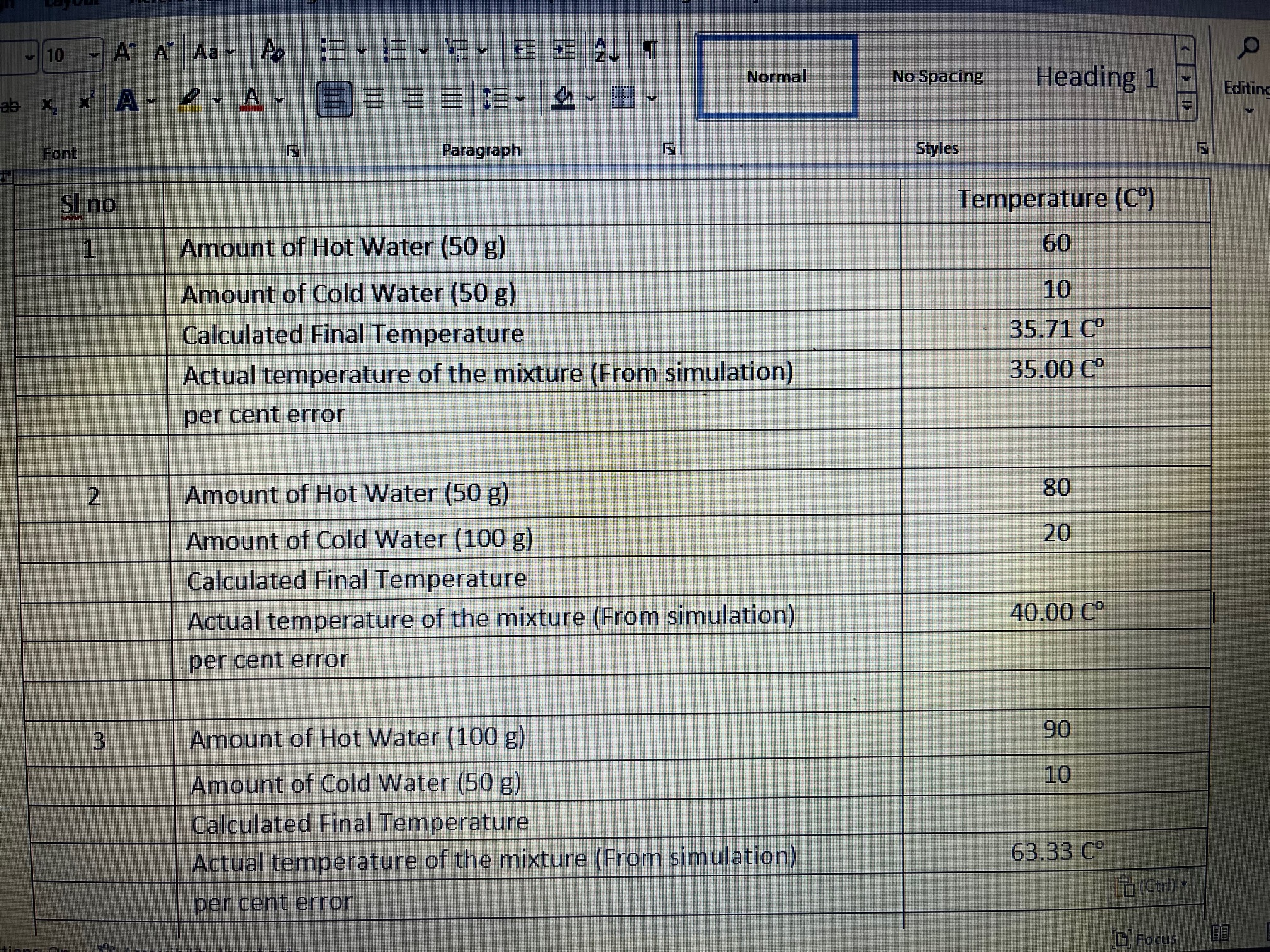
Voice Editor Determine the Calculated Final Temperature by using the equation learned in the lecture TF mn Th +mcTe (mc+mn) mi= mass of the hot water me= mass of the cold water Th= Temperature of the hot water To= Temperature of the cold water T= Temperature at the equilibrium (Final Temperature) Find out the percentage difference by considering calculated value as theoretical value and actual value from the simulation as experimental value experimental value -theoritical value percentage error= x 100% theoritical value- 10 ~ A" A" Aa ~ Ao Normal No Spacing E Heading 1 Editin Font Paragraph Styles SI no Temperature (C') Amount of Hot Water (50 g) 60 Amount of Cold Water (50 g) 10 Calculated Final Temperature 35.71 Co Actual temperature of the mixture (From simulation) 35.00 Co per cent error 2 Amount of Hot Water (50 g) 80 Amount of Cold Water (100 g) 20 Calculated Final Temperature Actual temperature of the mixture (From simulation) 40.00 Co per cent error Amount of Hot Water (100 g) 90 Amount of Cold Water (50 g) 10 Calculated Final Temperature Actual temperature of the mixture (From simulation) 63.33 C per cent error Lo (Ctl) ~ Focus
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


