Question: water has a high boiling point for its relatively low mass. complete the sentences to explain why Reset Help bent Water has a molecular geometry
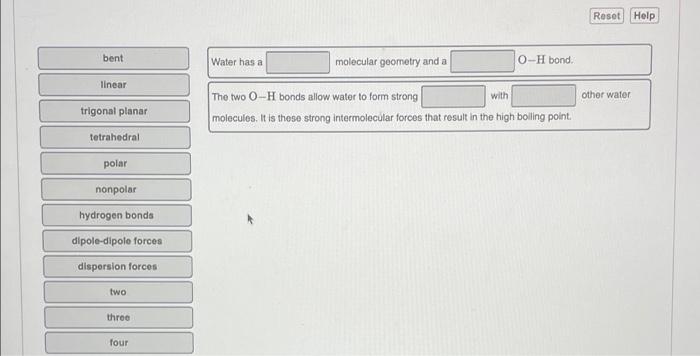
Reset Help bent Water has a molecular geometry and a O-H bond linear with other water trigonal planer The two 0-H bonds allow water to form strong molecules. It is thoso strong intermolecular forces that result in the high boling point tetrahedral polar nonpolar hydrogen bonds dipol-dipole forces dispersion forces two three four
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


