Question: We have seen that at the high temperature limit, where the number of units of energy is much greater than the number of degrees
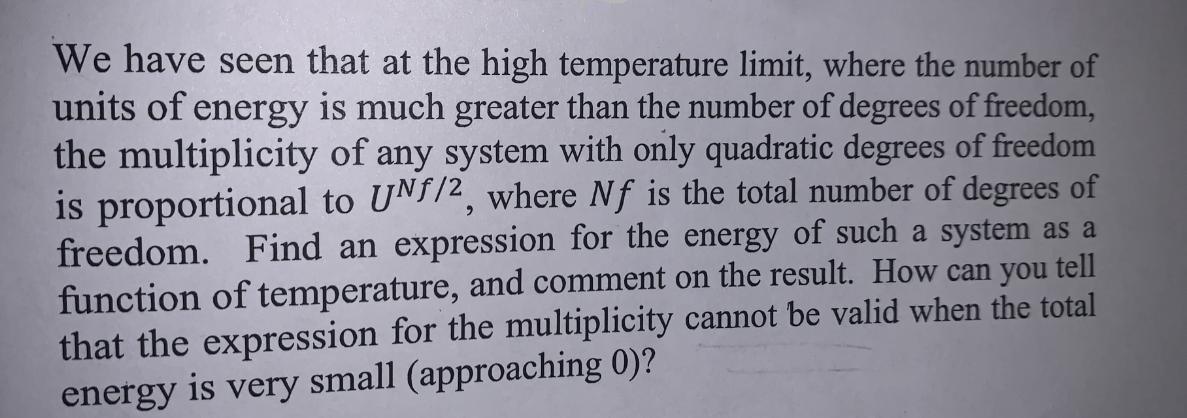
We have seen that at the high temperature limit, where the number of units of energy is much greater than the number of degrees of freedom, the multiplicity of any system with only quadratic degrees of freedom is proportional to UNf/2, where Nf is the total number of degrees of freedom. Find an expression for the energy of such a system as a function of temperature, and comment on the result. How can you tell that the expression for the multiplicity cannot be valid when the total energy is very small (approaching 0)?
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


