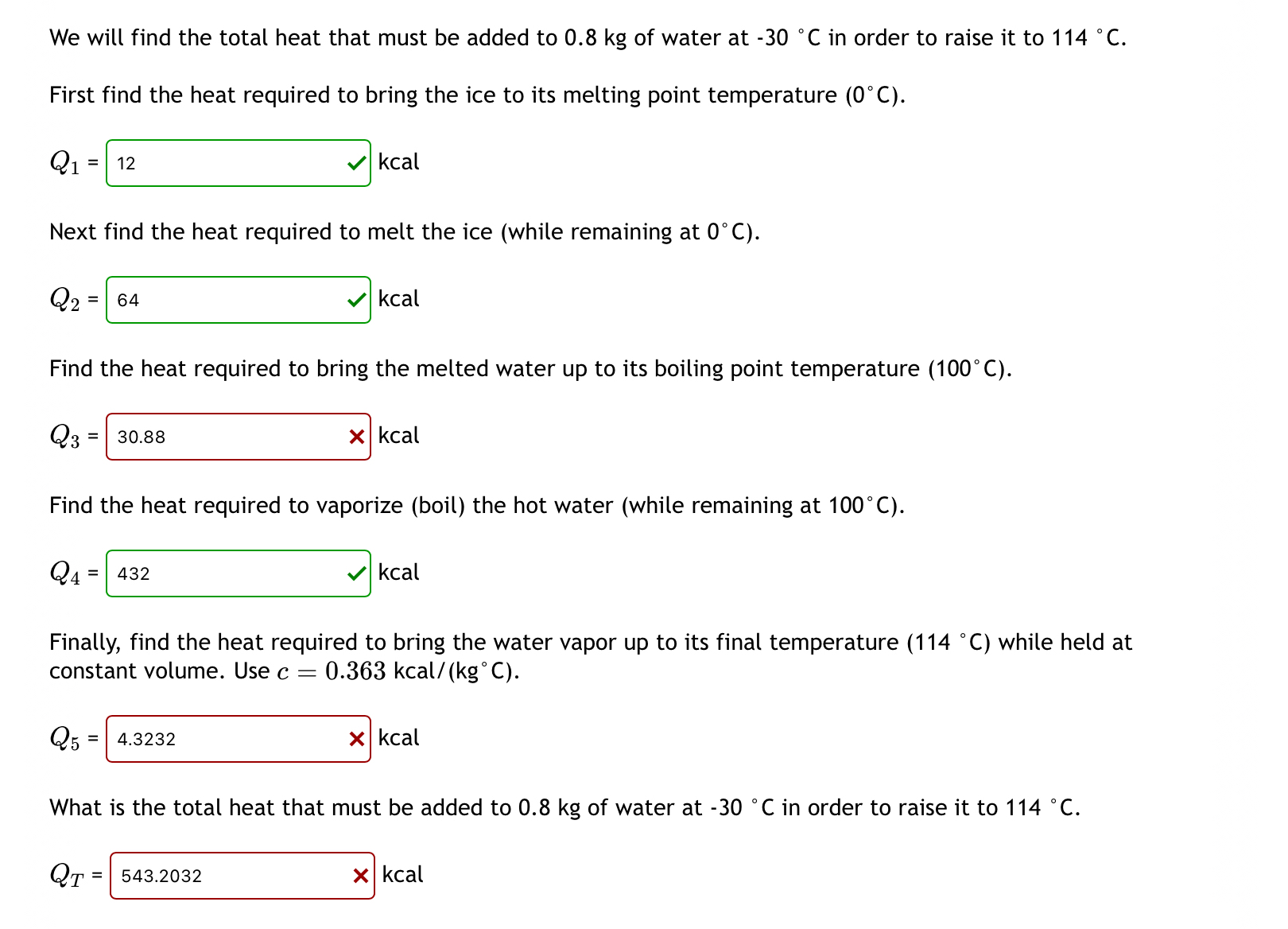
Question: We will find the total heat that must be added to 0 . 8 kg of water at - 3 0 C in order to
We will find the total heat that must be added to kg of water at in order to raise it to
First find the heat required to bring the ice to its melting point temperature
kcal
Next find the heat required to melt the ice while remaining at
kcal
Find the heat required to bring the melted water up to its boiling point temperature
kcal
Find the heat required to vaporize boil the hot water while remaining at
kcal
Finally, find the heat required to bring the water vapor up to its final temperature while held at constant volume. Use kca
kcal
What is the total heat that must be added to kg of water at in order to raise it to
kcal
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


