Question: We will use the atmospheric pressure data set from Exercise #1. Create a table in which you report the average atmospheric pressure with its uncertainty
We will use the atmospheric pressure data set from Exercise #1. Create a table in which you report the average atmospheric pressure with its uncertainty (using the proper number of significant figures) five different times. This table should report the pressure using the uncertainties calculated at 68%, 75%, 90%, 95% and 99% confidence limits. Remember that all reported numbers must have units and an appropriate number of significant figures.

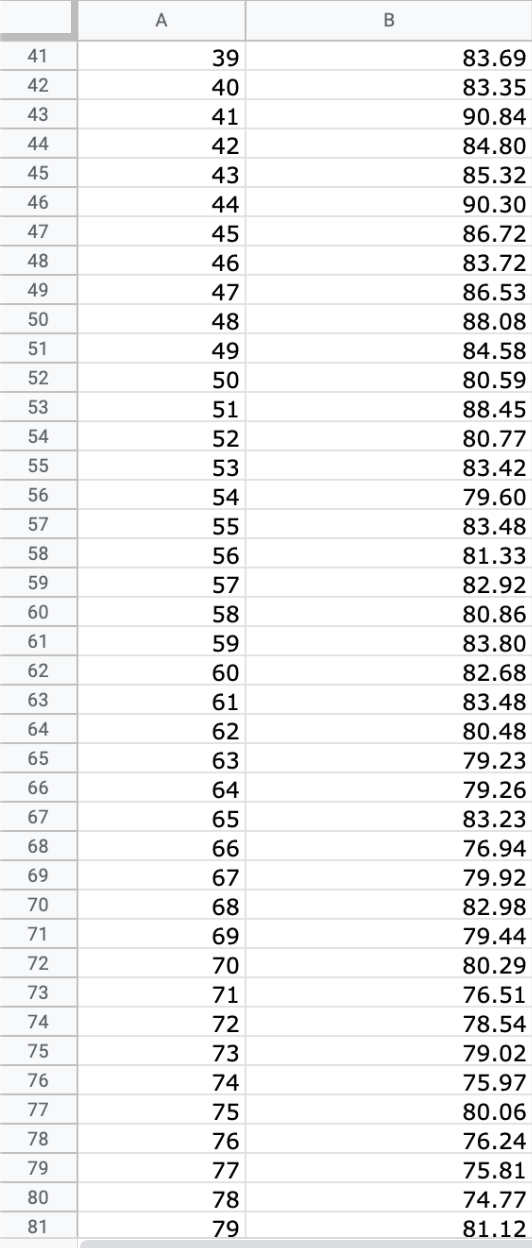
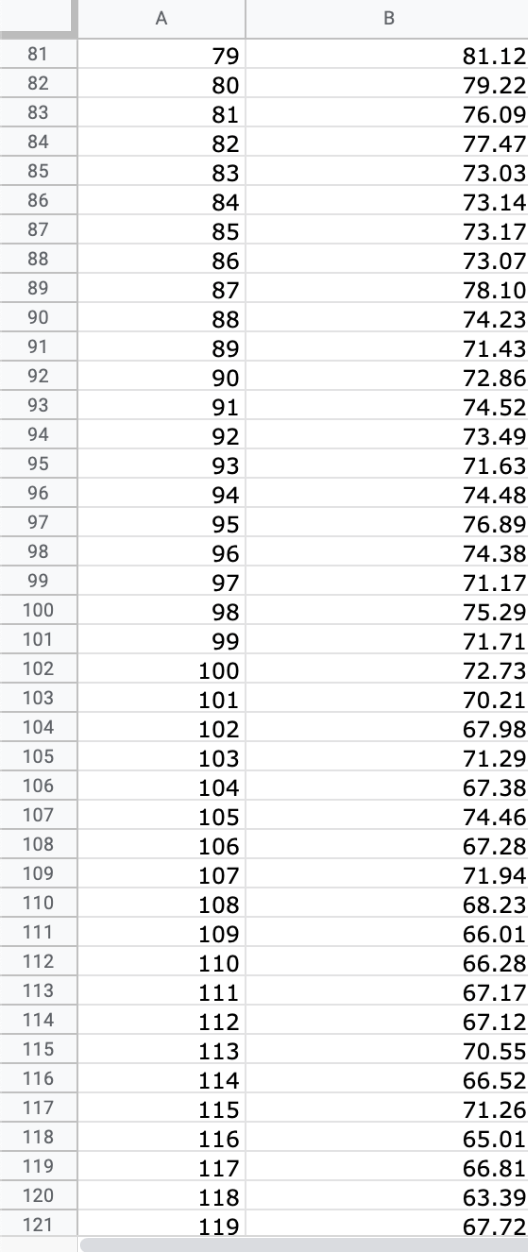
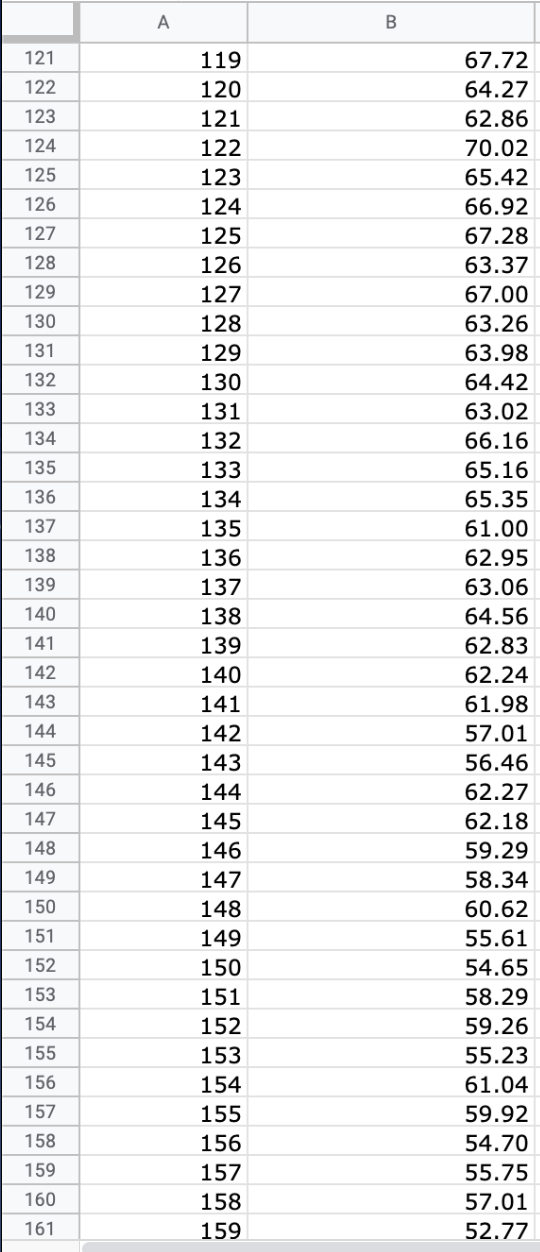
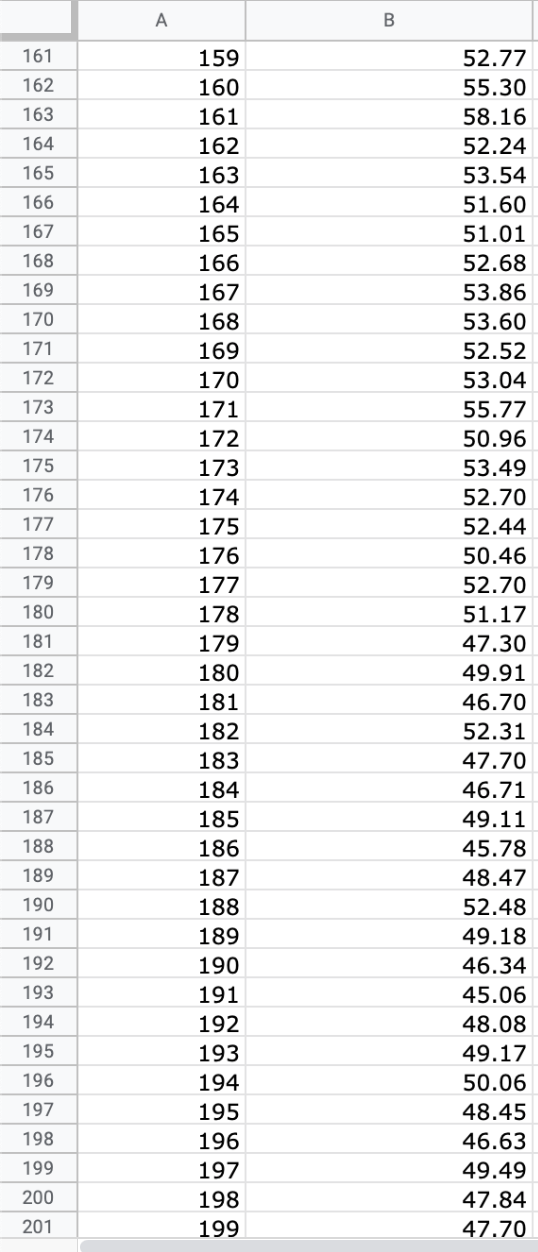
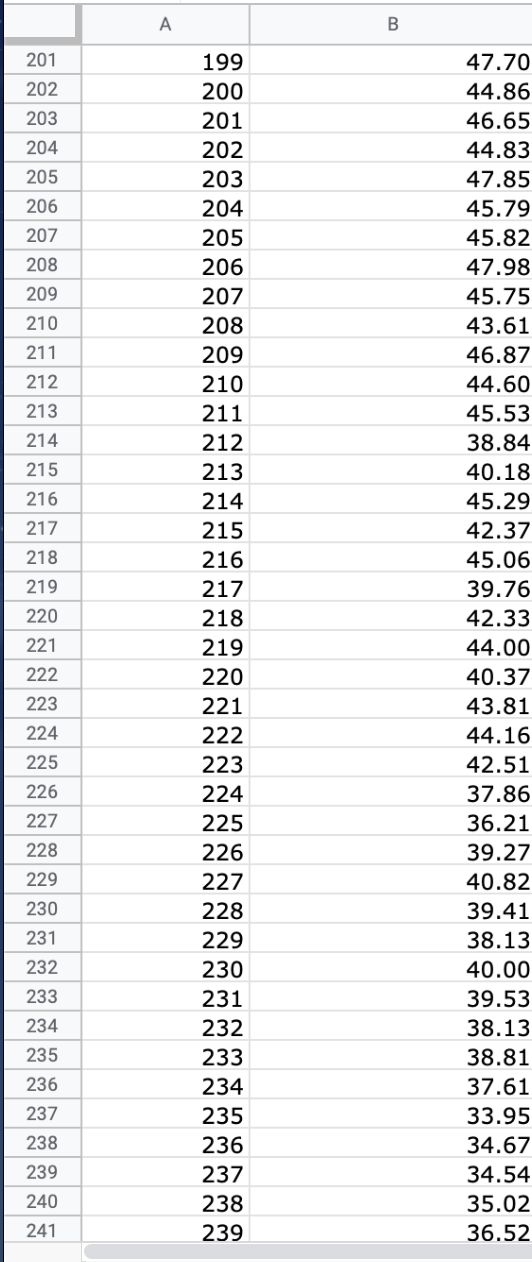
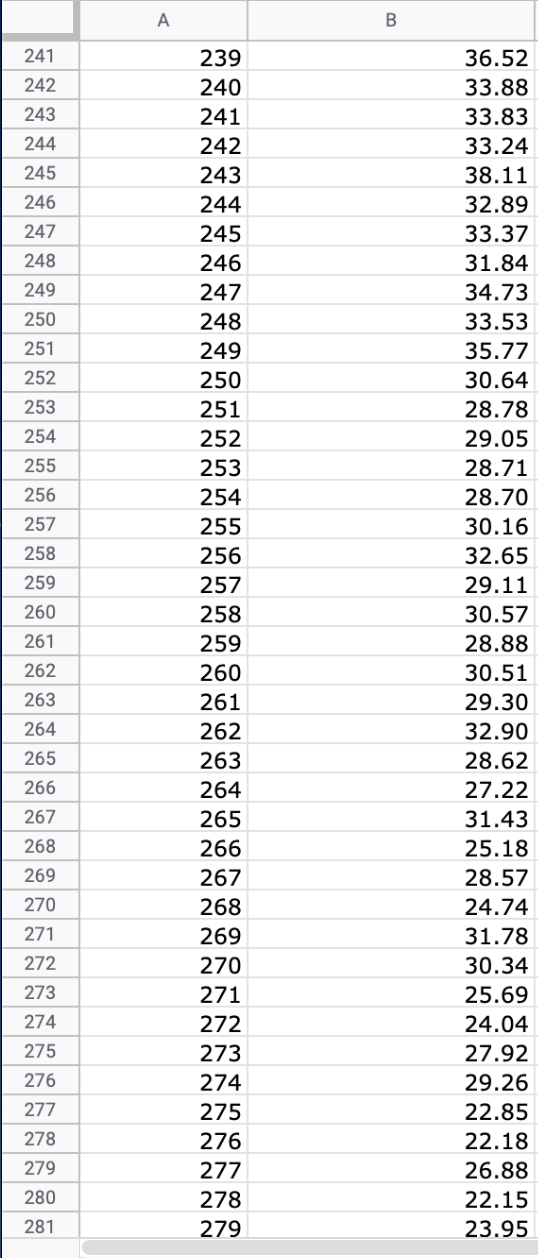
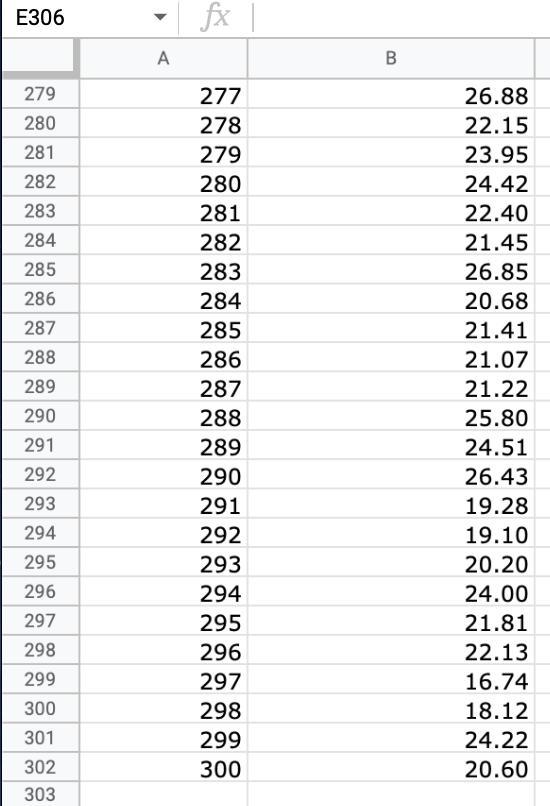
\begin{tabular}{|c|c|c|} \hline E306 & fX & \\ \hline & A & B \\ \hline 279 & 277 & 26.88 \\ \hline 280 & 278 & 22.15 \\ \hline 281 & 279 & 23.95 \\ \hline 282 & 280 & 24.42 \\ \hline 283 & 281 & 22.40 \\ \hline 284 & 282 & 21.45 \\ \hline 285 & 283 & 26.85 \\ \hline 286 & 284 & 20.68 \\ \hline 287 & 285 & 21.41 \\ \hline 288 & 286 & 21.07 \\ \hline 289 & 287 & 21.22 \\ \hline 290 & 288 & 25.80 \\ \hline 291 & 289 & 24.51 \\ \hline 292 & 290 & 26.43 \\ \hline 293 & 291 & 19.28 \\ \hline 294 & 292 & 19.10 \\ \hline 295 & 293 & 20.20 \\ \hline 296 & 294 & 24.00 \\ \hline 297 & 295 & 21.81 \\ \hline 298 & 296 & 22.13 \\ \hline 299 & 297 & 16.74 \\ \hline 300 & 298 & 18.12 \\ \hline 301 & 299 & 24.22 \\ \hline 302 & 300 & 20.60 \\ \hline 303 & 290 & 20 \\ \hline \end{tabular} \begin{tabular}{|c|c|c|} \hline E306 & fX & \\ \hline & A & B \\ \hline 279 & 277 & 26.88 \\ \hline 280 & 278 & 22.15 \\ \hline 281 & 279 & 23.95 \\ \hline 282 & 280 & 24.42 \\ \hline 283 & 281 & 22.40 \\ \hline 284 & 282 & 21.45 \\ \hline 285 & 283 & 26.85 \\ \hline 286 & 284 & 20.68 \\ \hline 287 & 285 & 21.41 \\ \hline 288 & 286 & 21.07 \\ \hline 289 & 287 & 21.22 \\ \hline 290 & 288 & 25.80 \\ \hline 291 & 289 & 24.51 \\ \hline 292 & 290 & 26.43 \\ \hline 293 & 291 & 19.28 \\ \hline 294 & 292 & 19.10 \\ \hline 295 & 293 & 20.20 \\ \hline 296 & 294 & 24.00 \\ \hline 297 & 295 & 21.81 \\ \hline 298 & 296 & 22.13 \\ \hline 299 & 297 & 16.74 \\ \hline 300 & 298 & 18.12 \\ \hline 301 & 299 & 24.22 \\ \hline 302 & 300 & 20.60 \\ \hline 303 & 290 & 20 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


