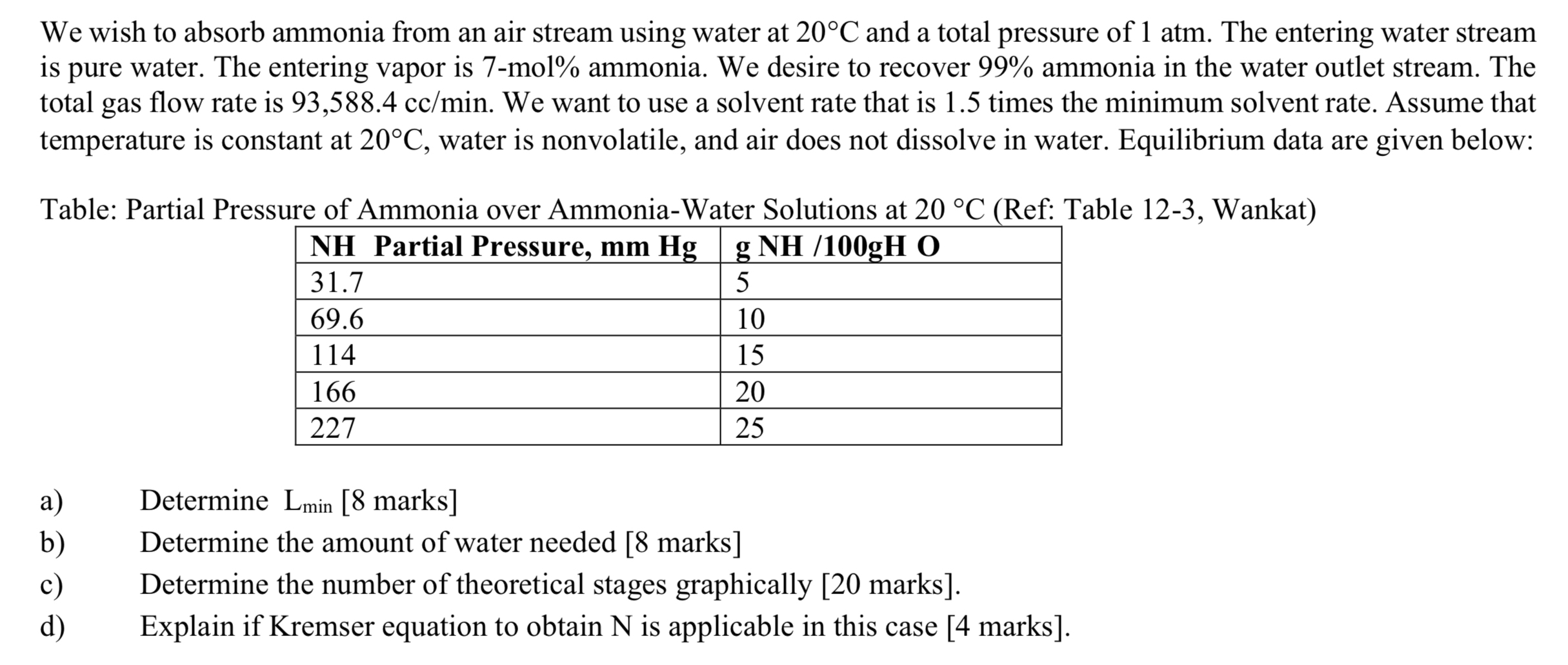
Question: We wish to absorb ammonia from an air stream using water at 2 0 C and a total pressure of 1 atm . The entering
We wish to absorb ammonia from an air stream using water at and a total pressure of atm The entering water stream is pure water. The entering vapor is mol ammonia. We desire to recover ammonia in the water outlet stream. The total gas flow rate is We want to use a solvent rate that is times the minimum solvent rate. Assume that temperature is constant at water is nonvolatile, and air does not dissolve in water. Equilibrium data are given below:
Table: Partial Pressure of Ammonia over AmmoniaWater Solutions at Ref: Table Wankat
tableNH Partial Pressure, O
a Determine marks
b Determine the amount of water needed marks
c Determine the number of theoretical stages graphically marks
d Explain if Kremser equation to obtain N is applicable in this case marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


