Question: We've already known that Fe+ + e Fe+, E-0.77v (1) Fe(OH)2 Fe+ + 2OH, Ksp1-6.010-5 (2) -> Fe(OH)3 Fe+ + 3OH, Ksp2=2.0X10-38 (3) Please
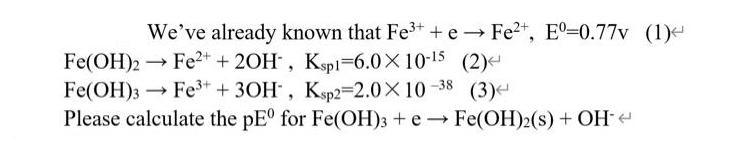
We've already known that Fe+ + e Fe+, E-0.77v (1) Fe(OH)2 Fe+ + 2OH, Ksp1-6.010-5 (2) -> Fe(OH)3 Fe+ + 3OH, Ksp2=2.0X10-38 (3) Please calculate the pE for Fe(OH)3 + e Fe(OH)2(s) + OH-
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Solution To calculate the pE for the given redox reaction we need to write the balanced halfreactions and their respective standard reduction potentials E Then we can calculate the overall standard cell potential Ecell and use the Nernst equation to find the cell potential Ecell at the given conditions Finally we can use the relation pE logEcell to find the pE The oxidation halfreaction ... View full answer

Get step-by-step solutions from verified subject matter experts


