Question: What are the half-reactions for the following reference electrodes? Include physical states. (a) The silver-silver chloride electrode. (b) The saturated calomel electrode. (c) What
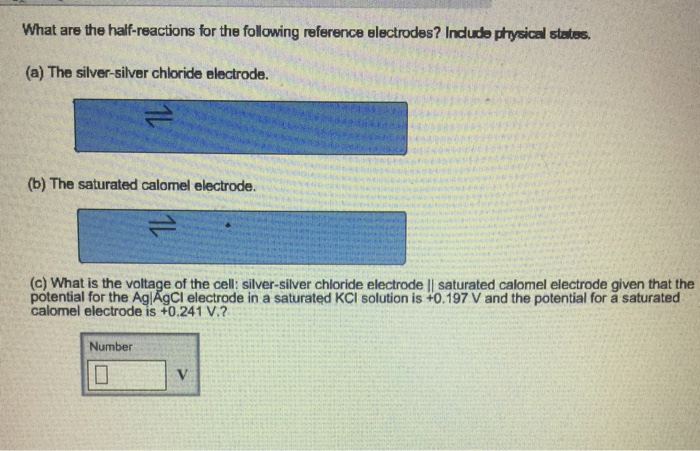
What are the half-reactions for the following reference electrodes? Include physical states. (a) The silver-silver chloride electrode. (b) The saturated calomel electrode. (c) What is the voltage of the cell: silver-silver chloride electrode || saturated calomel electrode given that the potential for the Ag|AgCl electrode in a saturated KCI solution is +0.197 V and the potential for a saturated calomel electrode is +0.241 V.? Number 0 V
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
a silversilver chloride electrode halfreacti... View full answer

Get step-by-step solutions from verified subject matter experts


