Question: What are the two methods most commonly used first when determining a rate law experimentally? method of initial rates method of multiple steady states perfect


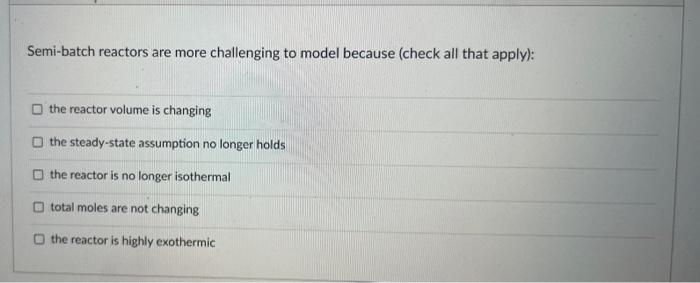
What are the two methods most commonly used first when determining a rate law experimentally? method of initial rates method of multiple steady states perfect mixing integral method method of natural logarithm linearization ideal gas assumption method of excess When modeling a system of reactions, rather than a single reaction, the primary modification to the reactor design equations are: O the reactor is not isothermal O total moles are changing, so you need the ideal gas law O steady-state assuraption no longer holds Othere are now multiple extents of reaction. Semi-batch reactors are more challenging to model because (check all that apply): the reactor volume is changing the steady-state assumption no longer holds the reactor is no longer isothermal total moles are not changing the reactor is highly exothermic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


