Question: What coefficient must be placed before H+(aq) in order to balance the following equation? *faq) + H+ (aq) + Cr2O72 aq) --> 6Fe3+(aq) + 2Cr3(aq)
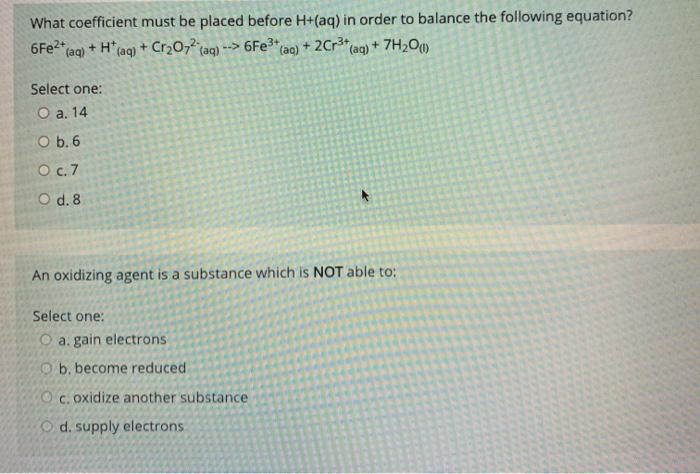
What coefficient must be placed before H+(aq) in order to balance the following equation? *faq) + H+ (aq) + Cr2O72 aq) --> 6Fe3+(aq) + 2Cr3(aq) + 7H2O) 6Fe2+ Select one: O a. 14 O b. 6 O c.7 O d. 8 An oxidizing agent is a substance which is NOT able to: Select one: O a. gain electrons b. become reduced Oc oxidize another substance d. supply electrons
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


