Question: what did i do wrong, and what would be the correct answer Based on the thermodynamic properties provided for water, determine the amount of energy
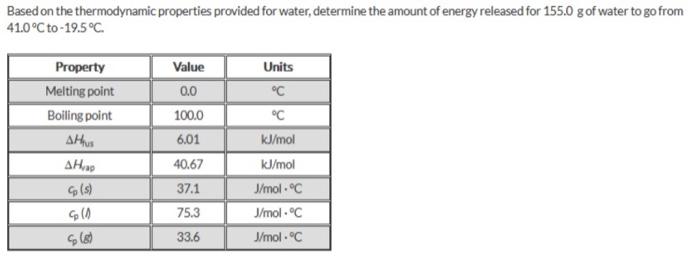

Based on the thermodynamic properties provided for water, determine the amount of energy released for 155.0g of water to go from 41.0C to 19.5C (1) Determine the amonl of energy reloased for 150.09 of H20 to go from 410c to 19.5c (1) a=mcT (2) a=nH&u (3) Q=MCT (1) Q=MCT=( Mass) (specific temp) (Change in temp) MassofH20=150Og(4)specifictemp=75.3J/molC(3)change=41.0c0.0c(150.0g)(75.3.1molc)(41.0C)=462095J (2) Q=nHfux=( Moles )( given) 150.0gH2O18.01528g1mol=8.326265259(8.3262)(6.01kJ/mol)k=1000x=50.04085421kJ (3) Q=H(T= (mass) (specifie temp) (change) Mass of H20=150Og specific temp. 37.1 ' 1mol. C (3) change=195C=0.0C(150.0g)(37.1)lm61&)(19.5)=1085175s (463,095k)4(50,04085421)+(108,5175) =521.5716458 =522kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


