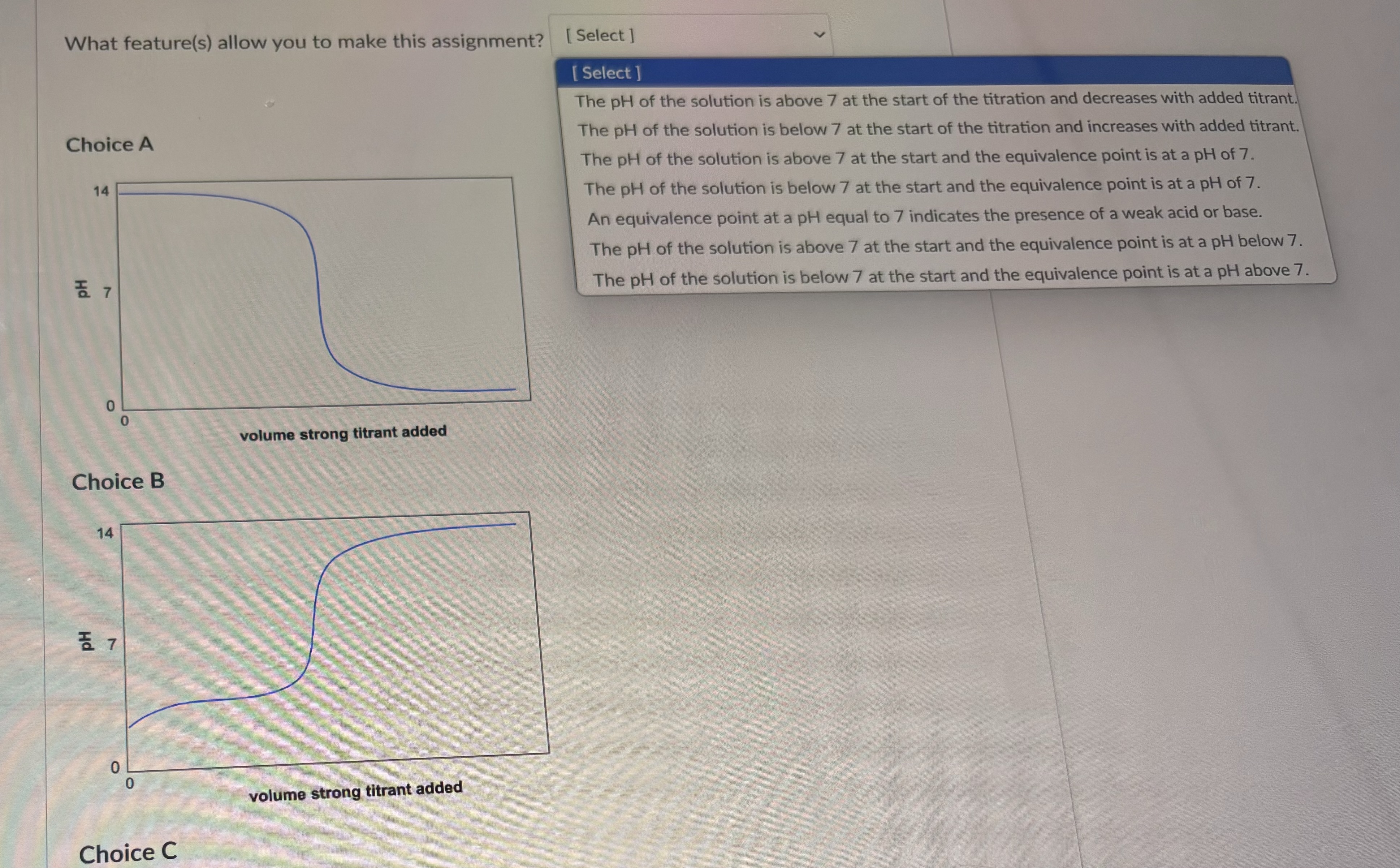
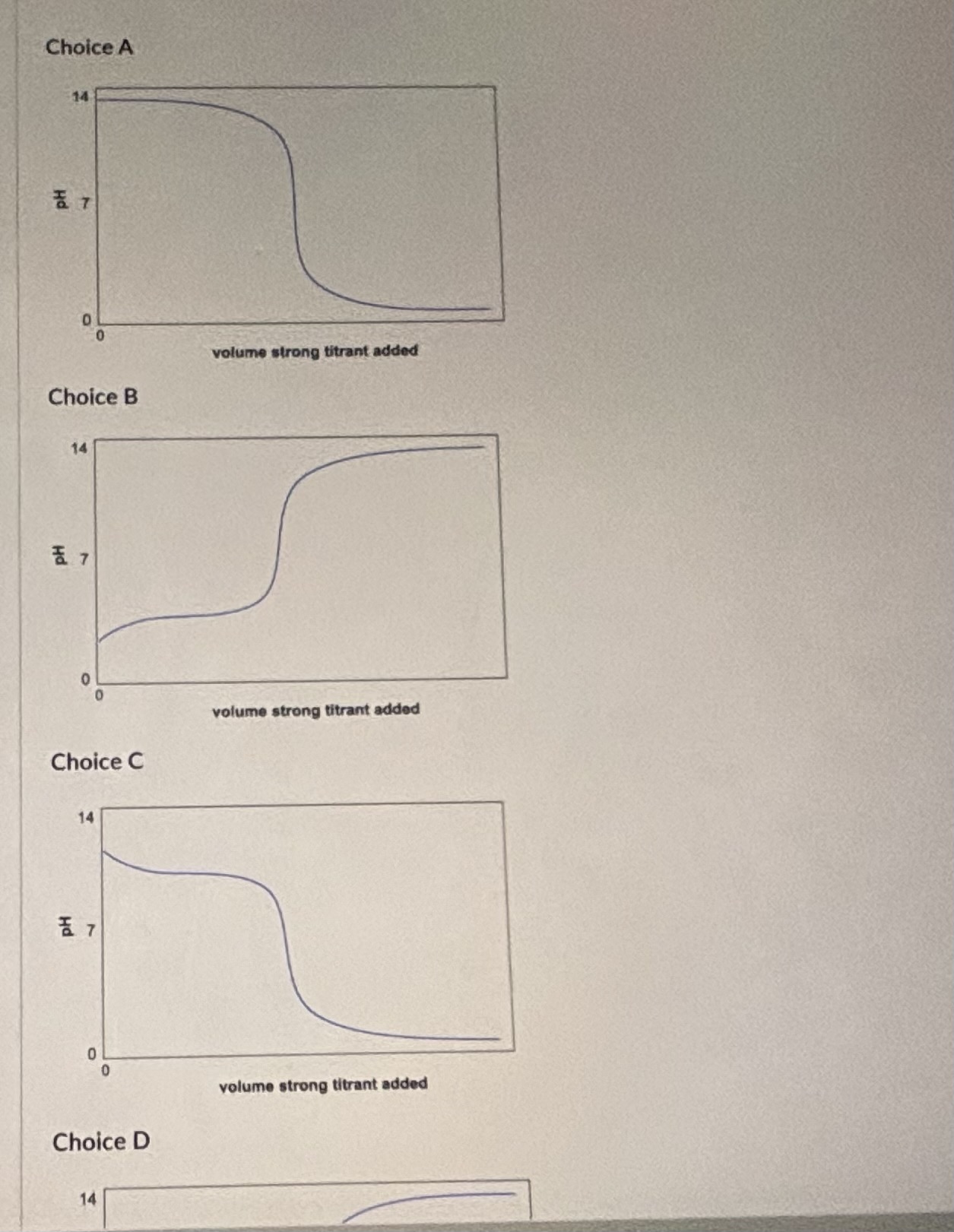
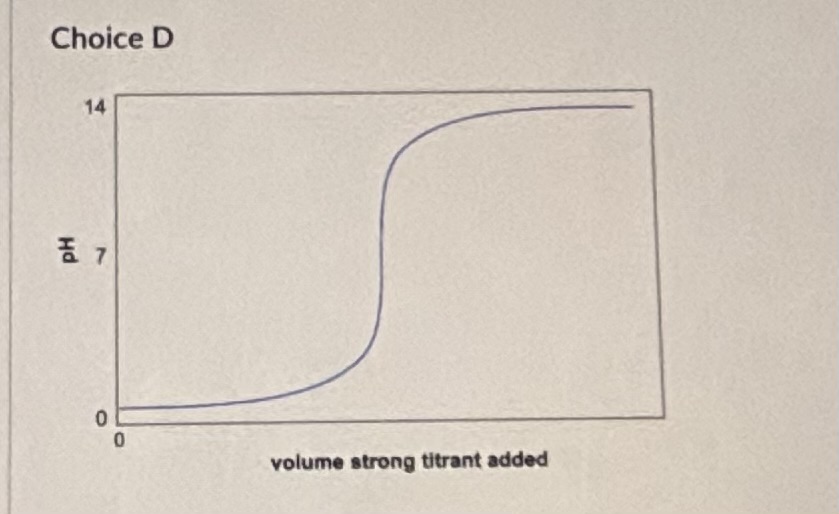
Question: What feature(s) allow you to make this assignment? [ Select ] [ Select ] The pH of the solution is above 7 at the start
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


