Question: What functional group(s) is/are present in the following compound? 1 amine and 2 amine amide and 2 amine 2 amine and nitrile nitrile and 1
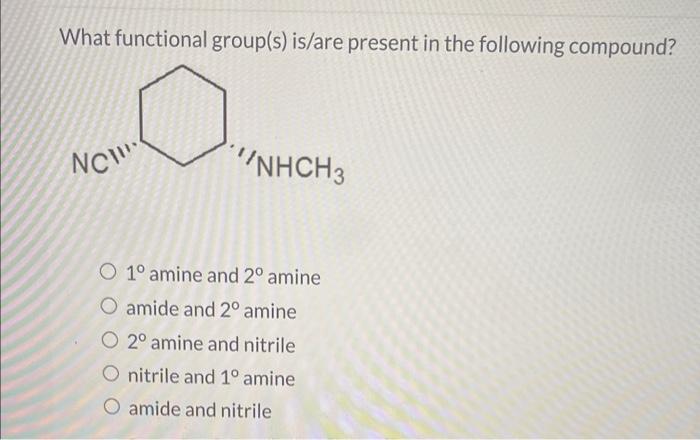
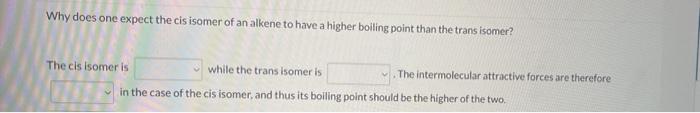
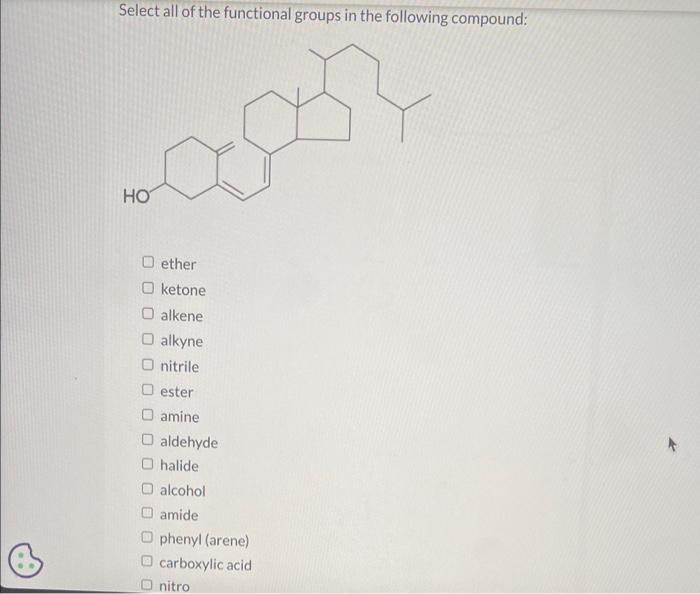
What functional group(s) is/are present in the following compound? 1 amine and 2 amine amide and 2 amine 2 amine and nitrile nitrile and 1 amine amide and nitrile Why does one expect the cis isomer of an alkene to have a higher boiling point than the trans isomer? The cis isomer is While the trans isomer is The intermolecular attractive forces are therefore in the case of the cis isomer. and thus its boiling point should be the higher of the two. Select all of the functional groups in the following compound: ether ketone alkene alkyne nitrile ester amine aldehyde halide alcohol amide phenyl (arene) carboxylic acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


