Question: what if we have a PFR reactor in part e? the design equation has an integral can you show how to solve I got a
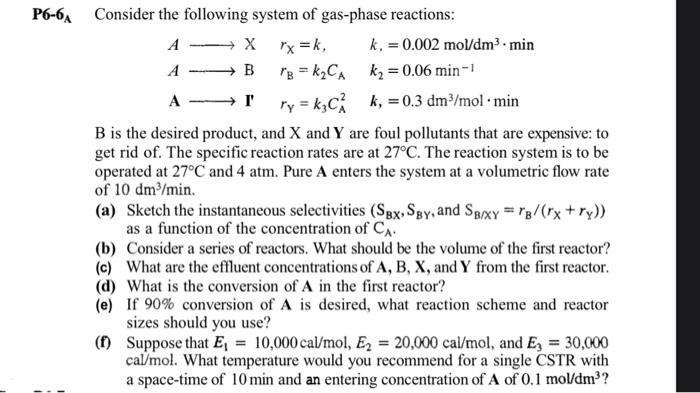
P6-6 AConsiderthefollowingsystemofgas-phasereactions: AXABAIrX=k,rB=k2CArY=k3CA2k,=0.002mol/dm3mink2=0.06min1k,=0.3dm3/molmin B is the desired product, and X and Y are foul pollutants that are expensive: to get rid of. The specific reaction rates are at 27C. The reaction system is to be operated at 27C and 4atm. Pure A enters the system at a volumetric flow rate of 10dm3/min. (a) Sketch the instantaneous selectivities (SBX,SBY, and SB/XY=rB/(rX+rY)) as a function of the concentration of CA. (b) Consider a series of reactors. What should be the volume of the first reactor? (c) What are the effluent concentrations of A,B,X, and Y from the first reactor. (d) What is the conversion of A in the first reactor? (e) If 90% conversion of A is desired, what reaction scheme and reactor sizes should you use? (f) Suppose that E1=10,000cal/mol,E2=20,000cal/mol, and E3=30,000 cal/mol. What temperature would you recommend for a single CSTR with a space-time of 10min and an entering concentration of A of 0.1mol/dm3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


