Question: 17. How likely is it to observe a bond angle of 20 or of 50 between the first three carbon atoms of 1 mole

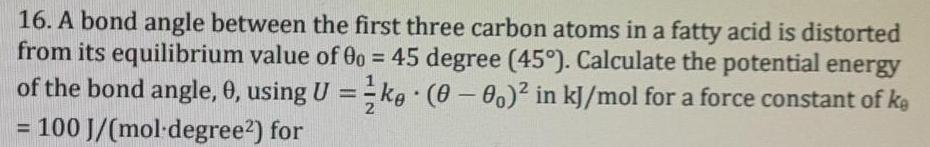
17. How likely is it to observe a bond angle of 20 or of 50 between the first three carbon atoms of 1 mole fatty acid from question 16 at room temperature? Hint: consider the equilibrium bond angle and the respective distorted bond angle as two thermodynamic states and calculate the ratio of both states at 298K. 16. A bond angle between the first three carbon atoms in a fatty acid is distorted from its equilibrium value of 00 = 45 degree (45). Calculate the potential energy of the bond angle, 0, using U =-kg (0-00)2 in kJ/mol for a force constant of ke %3D %3D 100 J/(mol-degree2) for %3D
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


