Question: What information more do you need????? Example Galvanic cell 8.17 Cell potential, equilibrium constant of chemical reaction Write the scheme of a galvanic cell consisting
 What information more do you need?????
What information more do you need?????
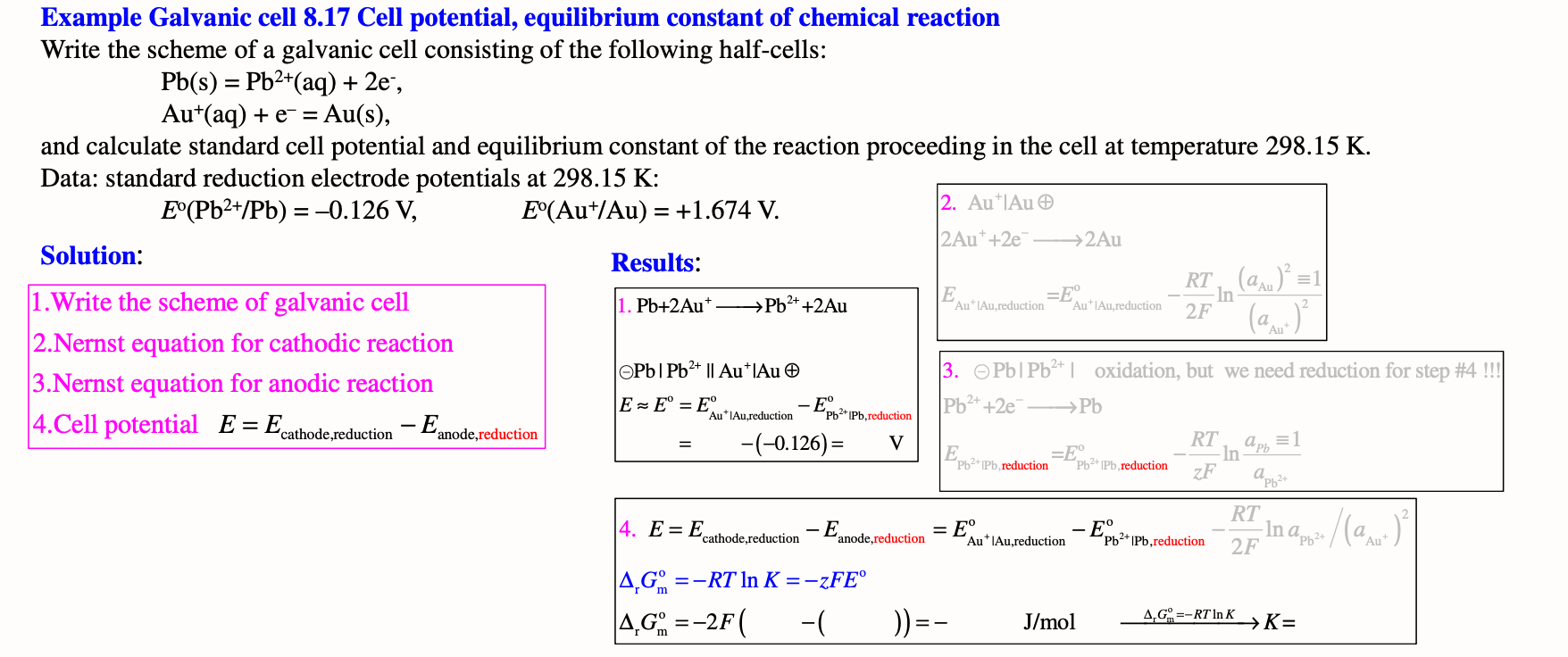
Example Galvanic cell 8.17 Cell potential, equilibrium constant of chemical reaction Write the scheme of a galvanic cell consisting of the following half-cells: Pb(s)=Pb2+(aq)+2eAu+(aq)+e=Au(s) and calculate standard cell potential and equilibrium constant of the reaction proceeding in the cell at temperature 298.15K. Data: standard reduction electrode potentials at 298.15K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


