Question: What is the answer to this question? How much gram of potassium dichromate [K2Cr2O7 molar mass = 294.2 g/mol] required to make 465 mL of
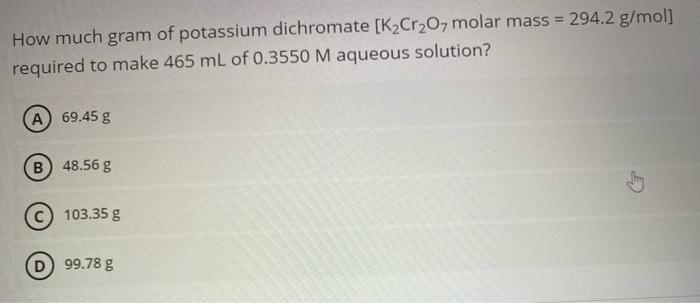
How much gram of potassium dichromate [K2Cr2O7 molar mass = 294.2 g/mol] required to make 465 mL of 0.3550 M aqueous solution? A 69.45 g B) 48.56 g 103.35 g 99.78 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


