Question: What is the bond angle around the indicated atom? 180 120 109.55 90 Is the indicated bond in the structure below a sigma or pi
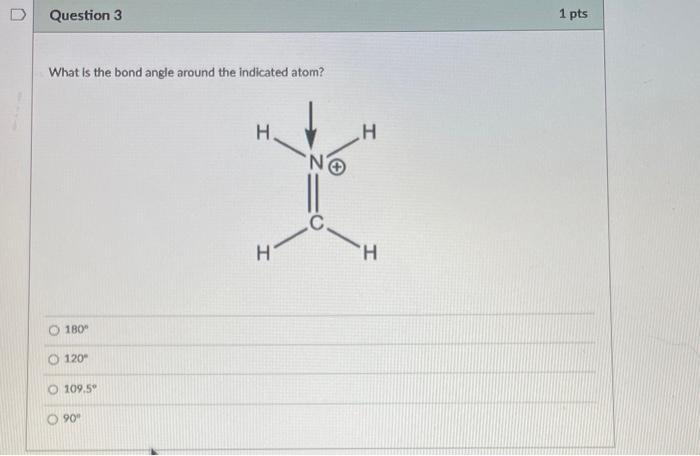
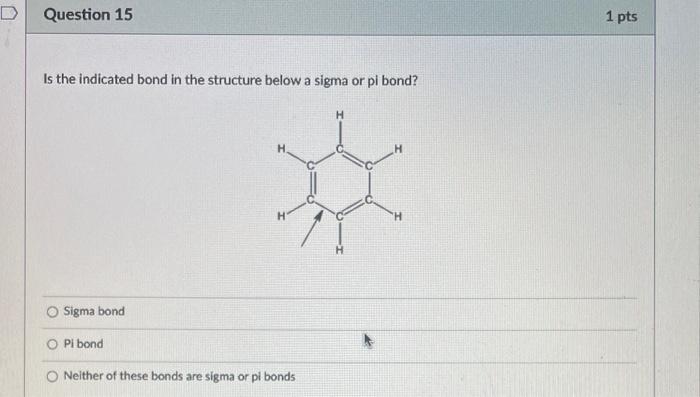
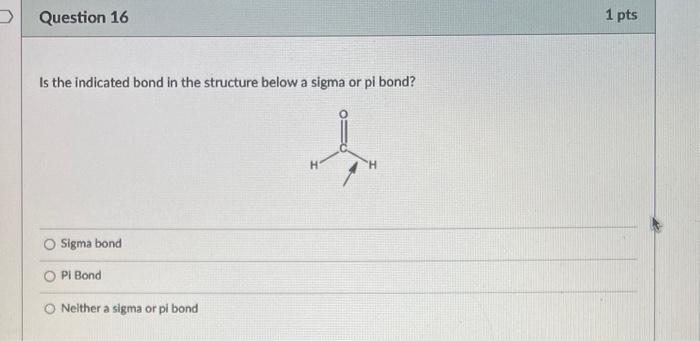
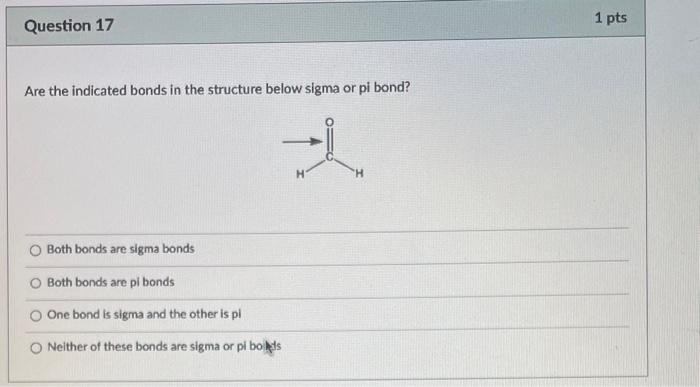
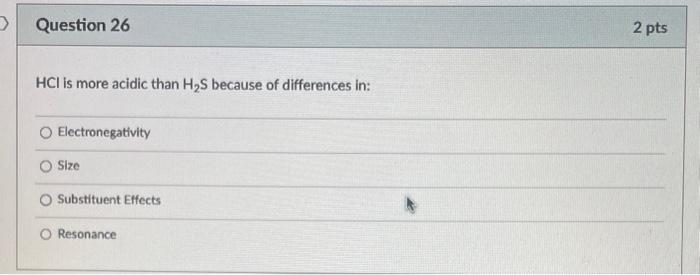
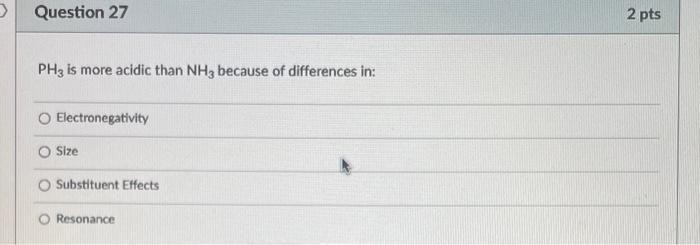
What is the bond angle around the indicated atom? 180 120 109.55 90 Is the indicated bond in the structure below a sigma or pi bond? Sigma bond Pibond Neither of these bonds are sigma or pi bonds Is the indicated bond in the structure below a sigma or pi bond? Sigma bond Pi Bond Nelther a sigma or pi bond Are the indicated bonds in the structure below sigma or pi bond? Both bonds are sigma bonds Both bonds are pi bonds One bond is sigma and the other is pi Neither of these bonds are sigma or pi bolys HCl is more acidic than H2S because of differences in: Electronegativity Size Substituent Effects Resonance PH3 is more acidic than NH3 because of differences in: Electronegativity Size Substituent Effects Resonance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


