Question: what is the right answer for q2 and q3 Question Completion Status: 10 points QUESTION 2 In an adiabatic mixer operating at 1 bar, 100
what is the right answer for q2 and q3 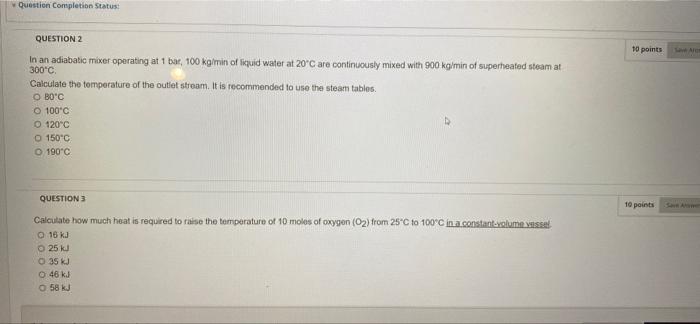

Question Completion Status: 10 points QUESTION 2 In an adiabatic mixer operating at 1 bar, 100 kg/min of squid water at 20C are continuously mixed with 900 kg/min of superheated steam at 300C Calculate the temperature of the outlet stroom. It is recommended to use the steam tables O BO'C O 100'c O 120C 150C O 190C QUESTION 3 10 points Calculate how much heat is required to raise the temperature of 10 moles of oxygen (O2) from 25C to 100C in a constant volumesse O 16 kJ 25 kJ O 35 kJ O 48 kJ 58 kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


