Question: what is the steps to get the answer shown? Use the following data for solving problems: Viscosity of methane ()=0.011103kgm1s1 Molecular weight (M)=16.0kgkmol1 Universal gas

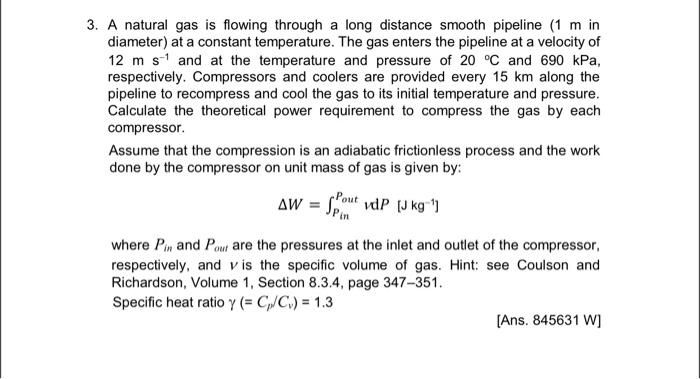
Use the following data for solving problems: Viscosity of methane ()=0.011103kgm1s1 Molecular weight (M)=16.0kgkmol1 Universal gas constant (R)=8314Jkmol1K1 3. A natural gas is flowing through a long distance smooth pipeline ( 1m in diameter) at a constant temperature. The gas enters the pipeline at a velocity of 12ms1 and at the temperature and pressure of 20C and 690kPa, respectively. Compressors and coolers are provided every 15km along the pipeline to recompress and cool the gas to its initial temperature and pressure. Calculate the theoretical power requirement to compress the gas by each compressor. Assume that the compression is an adiabatic frictionless process and the work done by the compressor on unit mass of gas is given by: W=PinPoutvdP[Jkg1] where Pin and Pout are the pressures at the inlet and outlet of the compressor, respectively, and v is the specific volume of gas. Hint: see Coulson and Richardson, Volume 1, Section 8.3.4, page 347-351. Specific heat ratio (=Cp/Cv)=1.3 [Ans. 845631W ]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


