Question: What more information is needed for the question? Question 6. Consider 1) the HMO analysis for a generic enolate (shown below). (2) the competitive alkylation
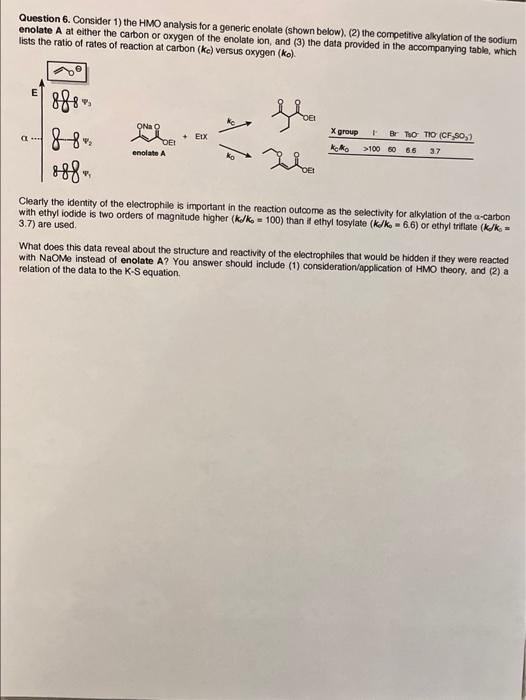
Question 6. Consider 1) the HMO analysis for a generic enolate (shown below). (2) the competitive alkylation of the sodium enolate A at either the carbon or oxygen of the enolate ion, and (3) the data provided in the accompanying table, which Tists the ratio of rates of reaction at carbon (ke) versus oxygen (ko) 900 E gloo ONA O 888 88 888 X group B Tho TIO (CF 90.2 >100 50 66 3.7 Acho enolate A Clearly the identity of the electrophile is important in the reaction outcome as the selectivity for alkylation of the a-carbon with ethyl iodide is two orders of magnitude higher (k/ks = 100) than it ethyl tosylate (k/k - 6.6) or ethyl triflate (k/k = 3.7) are used What does this data reveal about the structure and reactivity of the electrophiles that would be hidden if they were reacted with NaOme instead of enolate A? You answer should include (1) consideration application of HMO theory, and (2) a relation of the data to the K-S equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


