Question: What the answer please thats all the information i have A stream of water vapor flowing at 2.28kg/s enters a process at 132.2C and 1atm
What the answer please
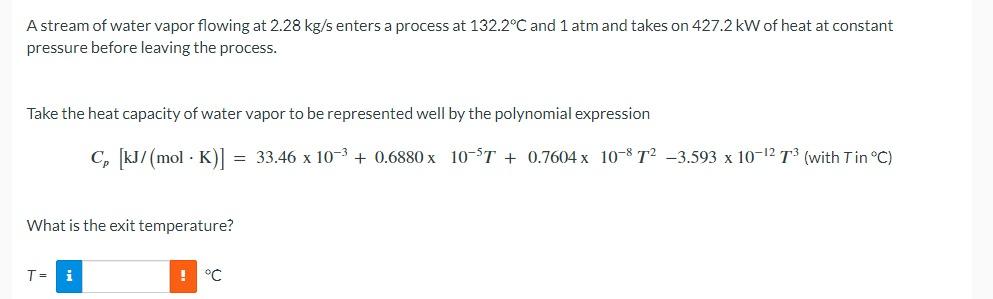
thats all the information i have
A stream of water vapor flowing at 2.28kg/s enters a process at 132.2C and 1atm and takes on 427.2kW of heat at constant pressure before leaving the process. Take the heat capacity of water vapor to be represented well by the polynomial expression Cp[kJ/(molK)]=33.46103+0.6880105T+0.7604108T23.5931012T3(withTinC) What is the exit temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


