Question: what would be the answer to part C? please explain! thankyou lots previous questions: Why? Under the nonstandard conditions the Gibbs energy change is less
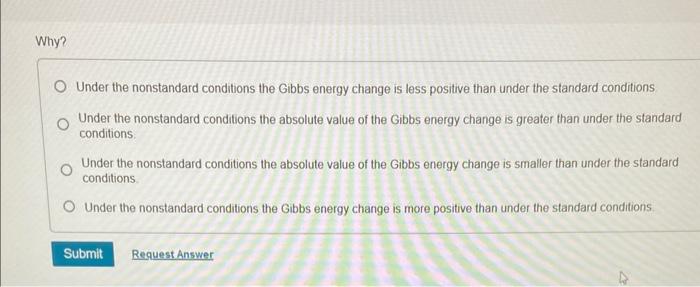
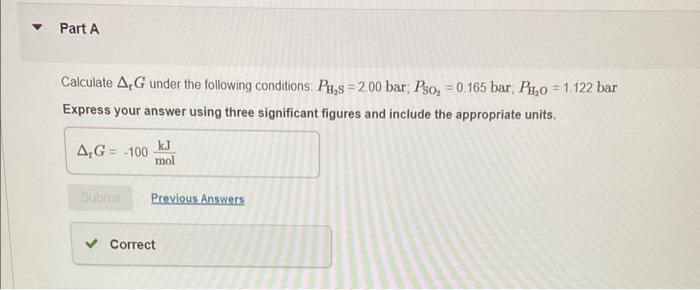
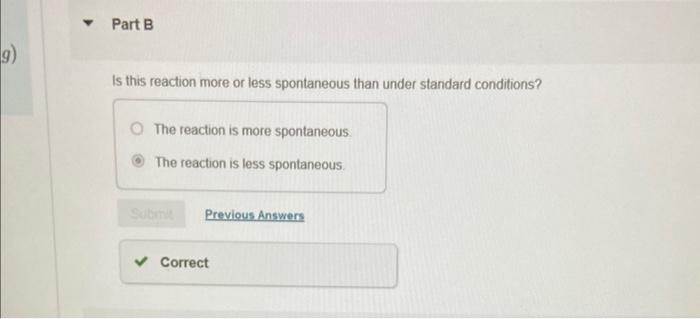
Why? Under the nonstandard conditions the Gibbs energy change is less positive than under the standard conditions Under the nonstandard conditions the absolute value of the Gibbs energy change is greater than under the standard conditions. Under the nonstandard conditions the absolute value of the Gibbs energy change is smaller than under the standard conditions. Under the nonstandard conditions the Gibbs energy change is more positive than under the standard conditions. Calculate rG under the following conditions: PH2S=2.00bar;PSO2=0.165bar,PH2O=1.122 bar Express your answer using three significant figures and include the appropriate units. Is this reaction more or less spontaneous than under standard conditions? The reaction is more spontaneous: The reaction is less spontaneous
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


