Question: Whatem (Marks =30 ) [Answer in the space provided, show important calculation steps] A 0.3kg of metal bar initially at 1200K is immersed in a
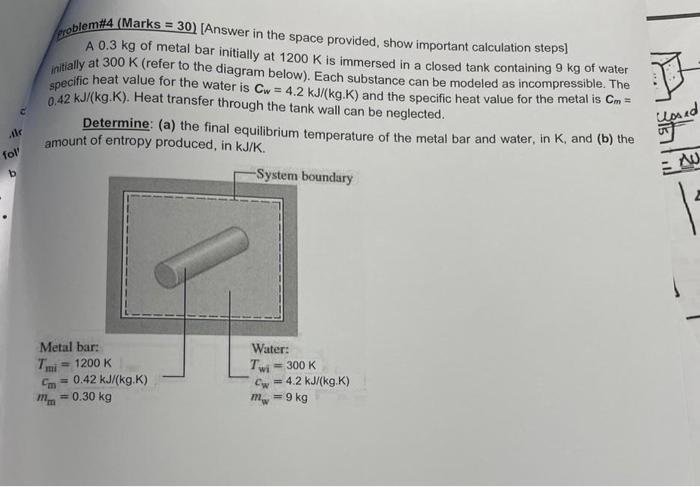
Whatem (Marks =30 ) [Answer in the space provided, show important calculation steps] A 0.3kg of metal bar initially at 1200K is immersed in a closed tank containing 9kg of water initially at 300K (refer to the diagram below). Each substance can be modeled as incompressible. The specific heat value for the water is Cw=4.2kJ/(kg.K) and the specific heat value for the metal is Cm= 0.42kJ/(kg.K). Heat transfer through the tank wall can be neglected. Determine: (a) the final equilibrium temperature of the metal bar and water, in K, and (b) the amount of entropy produced, in kJ/K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


