Question: When 5.58gH2 react by the following balanced equation, 37.5gHH2O are formed. What is the percent yield of the reaction? 2H2(g)+O2(g)2H2O(l) Select the correct answer below:
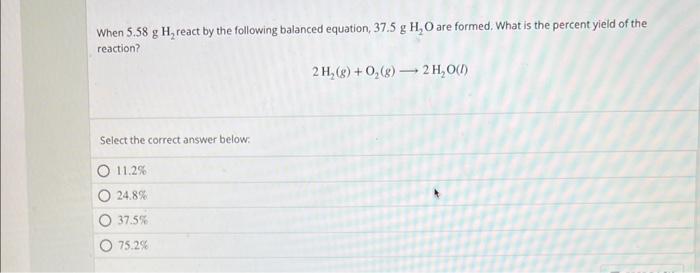
When 5.58gH2 react by the following balanced equation, 37.5gHH2O are formed. What is the percent yield of the reaction? 2H2(g)+O2(g)2H2O(l) Select the correct answer below: 11.2% 24.8% 37.5% 75.2%
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


