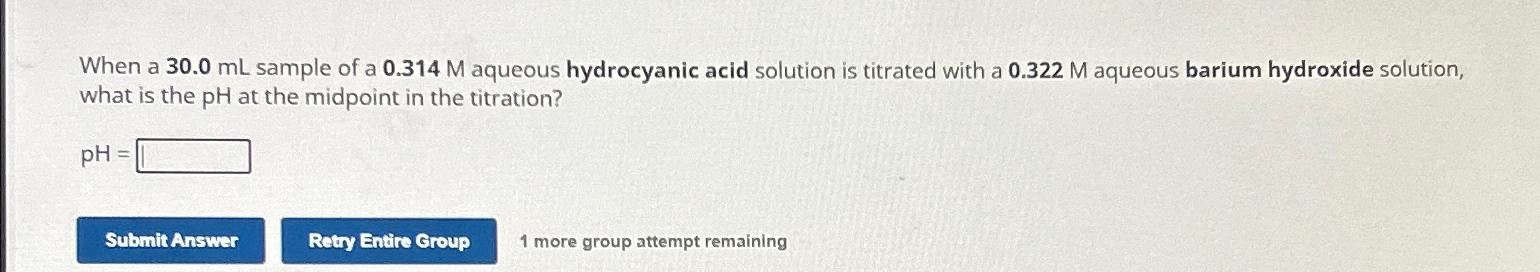
Question: When a 3 0 . 0 m L sample of a 0 . 3 1 4 M aqueous hydrocyanic acid solution is titrated with a
When a sample of a aqueous hydrocyanic acid solution is titrated with a aqueous barium hydroxide solution, what is the at the midpoint in the titration?
more group attempt remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


