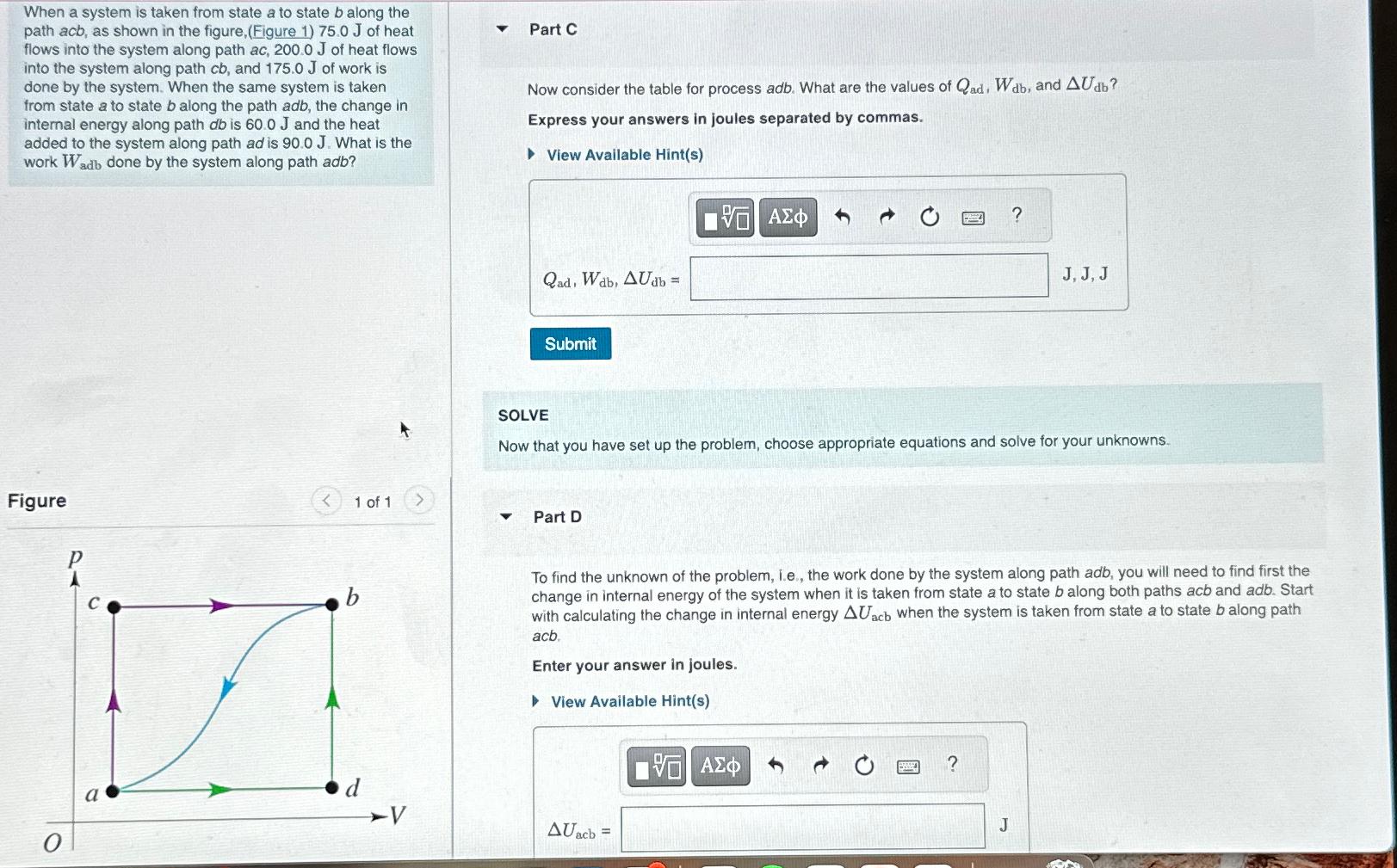
Question: When a system is taken from state a to state b along the path acb, as shown in the figure, ( Figure 1 ) 7
When a system is taken from state to state along the path acb, as shown in the figure,Figure of heat flows into the system along path of heat flows into the system along path and of work is done by the system. When the same system is taken from state to state along the path adb, the change in internal energy along path is and the heat added to the system along path ad is What is the work done by the system along path adb
Part C
Now consider the table for process adb. What are the values of and
Express your answers in joules separated by commas.
View Available Hints
SOLVE
Now that you have set up the problem, choose appropriate equations and solve for your unknowns.
Part D
To find the unknown of the problem, ie the work done by the system along path adb, you will need to find first the change in internal energy of the system when it is taken from state a to state along both paths acb and adb. Start with calculating the change in internal energy when the system is taken from state to state along path acb.
Enter your answer in joules.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


