Question: When aqueous solutions of barium nitrate and ammonium sulfate are mixed, the balance equation for this reaction is: Ba(NO3)2 (aq) + (NH4)2SO4 (aq)------ -

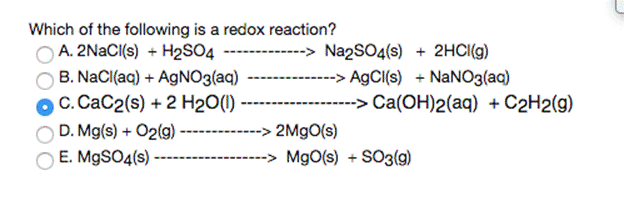

When aqueous solutions of barium nitrate and ammonium sulfate are mixed, the balance equation for this reaction is: Ba(NO3)2 (aq) + (NH4)2SO4 (aq)------ - 2 NH4NO3 (aq) + BaSO4(s) What are the "spectator ions" in the reaction? O Ba2+ and NO3" O NH4* and NO3 O so42- and NO3 NH4+ and SO42- O Ba2+ and SO42- Which of the following is a redox reaction? A. 2NACI{s) + H2S04 ---- B. NaCl(aq) + AGNO3(aq) OC. CaC2(s) + 2 H2O(1) D. Mg(s) + O2(g) E. M9SO4(s) -> Na2SO4(s) + 2HCI(g) --> A9CI(s) + NaNO3(aq) --> Ca(OH)2(aq) + C2H2(g) 2M9O(s) -> MgO(s) + SO3(9) 2.5000 g of KNO3 is dissolved in 200.0 ml aqueous solution that has density of 1.034 g/mL. Calculate for KNO3 in the solution: Do not write down the answers in exponential form molality 0.0022
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Q1 spectator ions will not form any preciptiate form the list NO3 is on bo... View full answer

Get step-by-step solutions from verified subject matter experts


