Question: When this compound is treated with (exposed to) strong base, which proton is removed first? Select an answer and submit. For keyboard navigation, use the
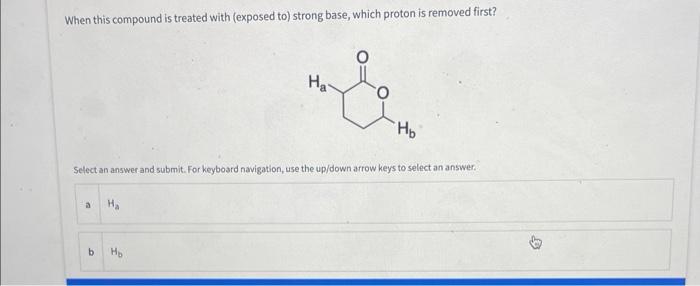
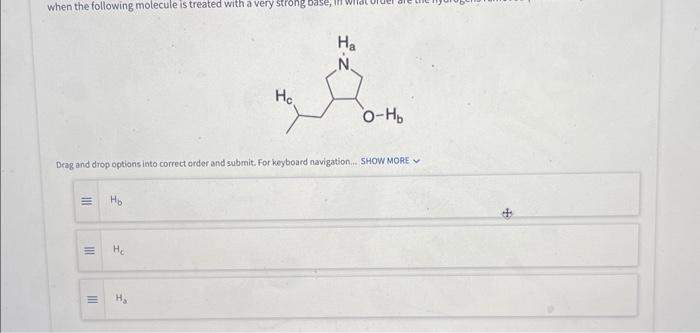
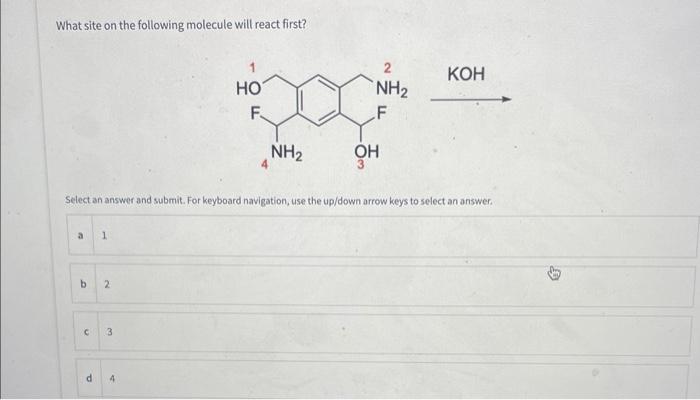
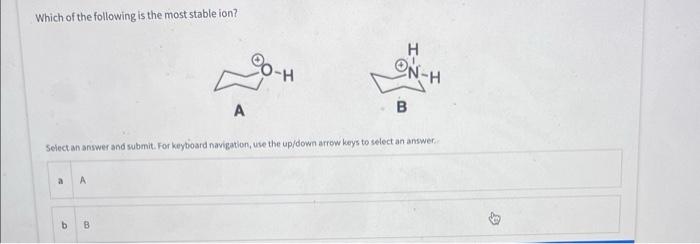
When this compound is treated with (exposed to) strong base, which proton is removed first? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. Deag and drop options into correct order and submit. For keyboard navigation.. SHOW MOAE V What site on the following molecule will react first? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. Which of the following is the most stable ion? Seiect an answer and wubmit. For keyboard navigation, une the up/down arrowkeys to select an answer. Why is the ion you chose in the above question the most stable? Select an answer and subemit. For keyboand owigation, use the up/down arrow keys to select an answer. a positive charge is on the most electronegative atom b positive charge is on the least electronegative atom c positive charge is on the largest atom a positive charge is on the smallest atom - effective charge is reduced the most by an inductive effect 1 effective charge is reduced the least by an inductive effect E charge is delocalized by resonance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


