Question: where am I going wrong either sig fig wise or calculation wise Photon Mutation of DNA Part I 1.0/1 point (graded) Physical constants that you
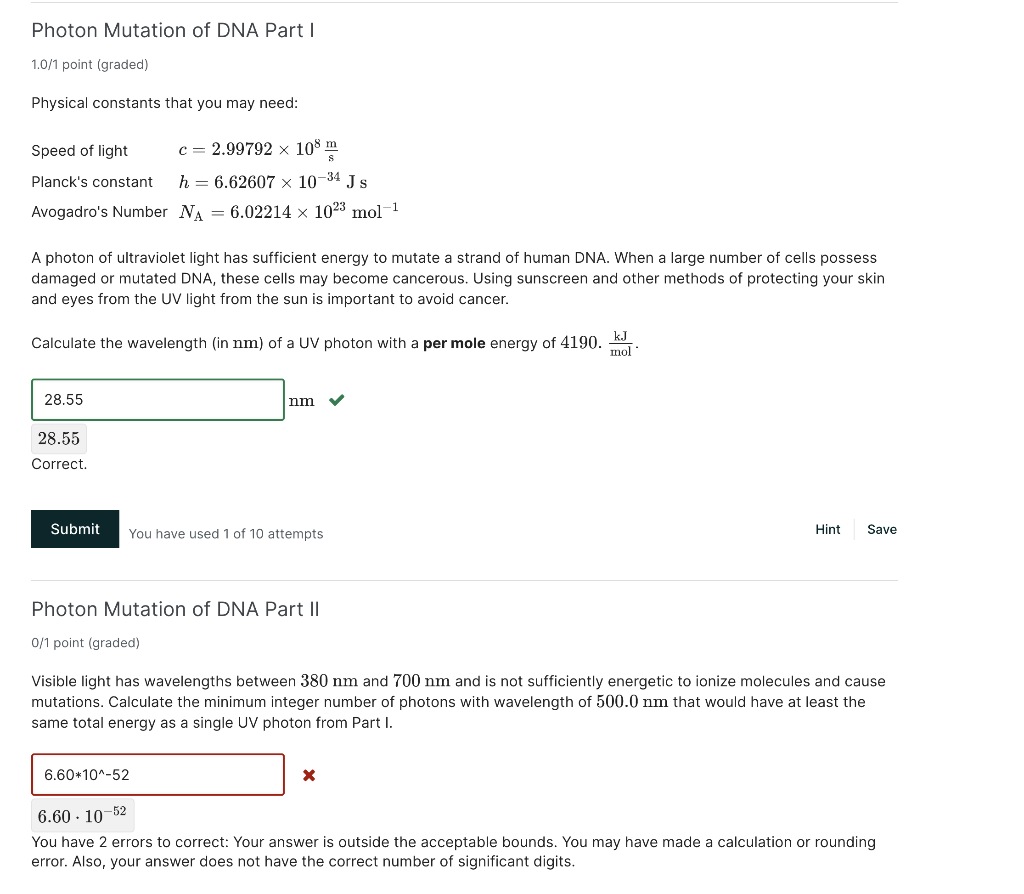 where am I going wrong either sig fig wise or calculation wise
where am I going wrong either sig fig wise or calculation wise
Photon Mutation of DNA Part I 1.0/1 point (graded) Physical constants that you may need: Speed of light c=2.99792108sm Planck's constant h=6.626071034Js Avogadro's Number NA=6.022141023mol1 A photon of ultraviolet light has sufficient energy to mutate a strand of human DNA. When a large number of cells possess damaged or mutated DNA, these cells may become cancerous. Using sunscreen and other methods of protecting your skin and eyes from the UV light from the sun is important to avoid cancer. Calculate the wavelength (in nm ) of a UV photon with a per mole energy of 4190.molkJ. 28.55 Correct. You have used 1 of 10 attempts Hint Save Photon Mutation of DNA Part II 0/1 point (graded) Visible light has wavelengths between 380nm and 700nm and is not sufficiently energetic to ionize molecules and cause mutations. Calculate the minimum integer number of photons with wavelength of 500.0nm that would have at least the same total energy as a single UV photon from Part I. 6.601052 You have 2 errors to correct: Your answer is outside the acceptable bounds. You may have made a calculation or rounding error. Also, your answer does not have the correct number of significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


