Question: WHI A3 Time and concentration data were collected for the reaction 0.52 A-products 0.484 0.44- 1(s) 0 20 [Al(M) 0.52 0.43 0.35 0.29 0.23 40
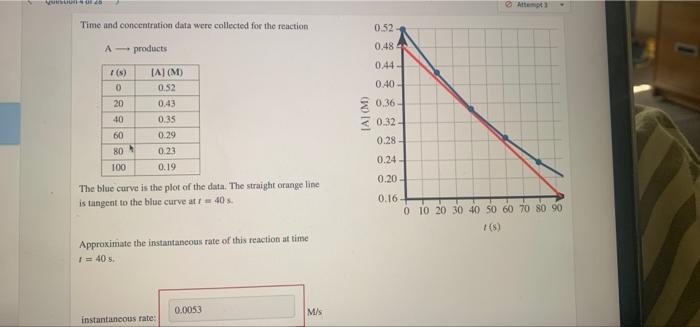
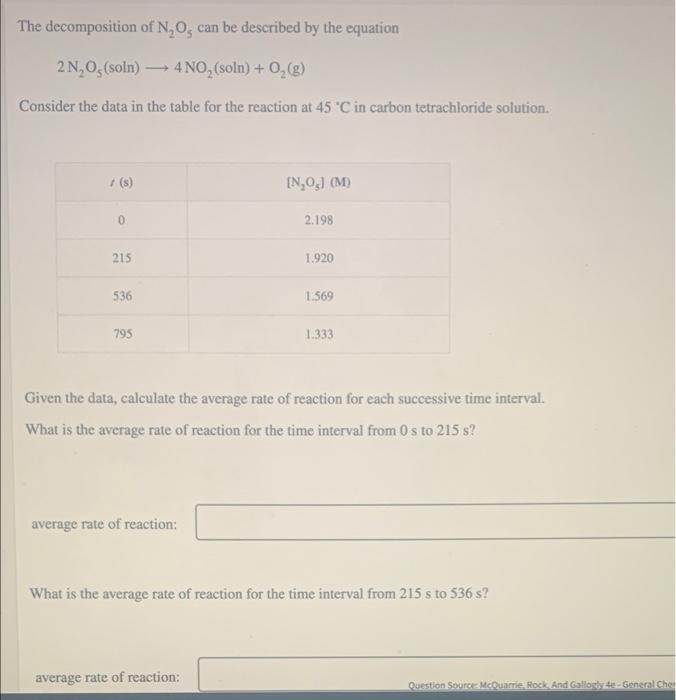

WHI A3 Time and concentration data were collected for the reaction 0.52 A-products 0.484 0.44- 1(s) 0 20 [Al(M) 0.52 0.43 0.35 0.29 0.23 40 8 8888 0.40 0.36 0.32-1 0.28 0.24 60 80 0.19 0.20 The blue curve is the plot of the data. The straight orange line is tangent to the blue curve at 40 0.16 0 10 20 30 40 50 60 70 80 90 1 (5) Approximate the instantaneous rate of this reaction at time 1 = 40 s. 0.0053 M/S instantaneous rate The decomposition of N, 0, can be described by the equation 2N, O (soln) 4 NO, (soln) + 0,(g) Consider the data in the table for the reaction at 45 C in carbon tetrachloride solution. + (8) [N,O) (M 0 2.198 215 1.920 536 1.569 795 1.333 Given the data, calculate the average rate of reaction for each successive time interval What is the average rate of reaction for the time interval from 0 s to 215 s? average rate of reaction: What is the average rate of reaction for the time interval from 215 s to 536 s? average rate of reaction: Question Source McQuarrie Rock And Gallos - General Cher What is the average rate of reaction for the time interval from 536 sto 795 s? M/S average rate of reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


