Question: which answer should be chosen? The reversible liquid reaction AB+C has the forward rate law rf=kfCA. Identify the correct expression for the overall rate of
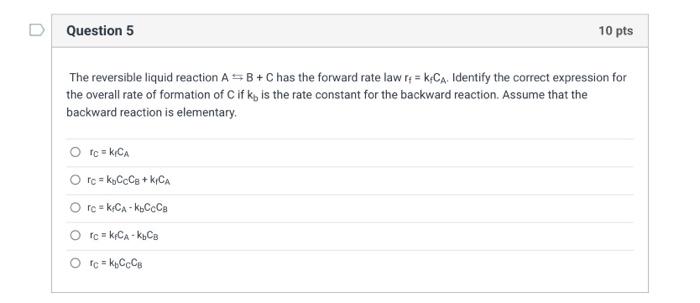
The reversible liquid reaction AB+C has the forward rate law rf=kfCA. Identify the correct expression for the overall rate of formation of C if kb is the rate constant for the backward reaction. Assume that the backward reaction is elementary. rC=k1CArC=kbCCCB+k1CArC=kfCAkbCCCBrC=kPCAkbCBrC=kBCCCB
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


