Question: which change in the system will NOT affect the equilibrium in the reaction below? Which change in the system will not affect the equilibrium in
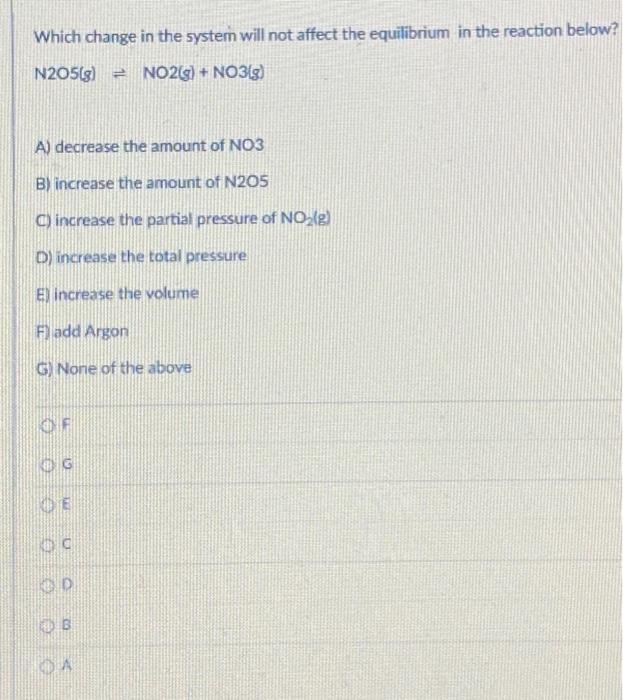
Which change in the system will not affect the equilibrium in the reaction below? N205[8] = NO2(g) + NO3(g) A) decrease the amount of NO3 B) increase the amount of N205 C) increase the partial pressure of NO(g) D) increase the total pressure El increase the volume F) add Argon G) None of the above OF OG DE B DA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


