Question: Which compound below represents a hydrocarbon? a) CH3CH2CH2NH2 b) CH3CH=CH2 c) CH3CH2CH2OH d) CH3CH2CH2F Which combination would you predict to not be soluble in each

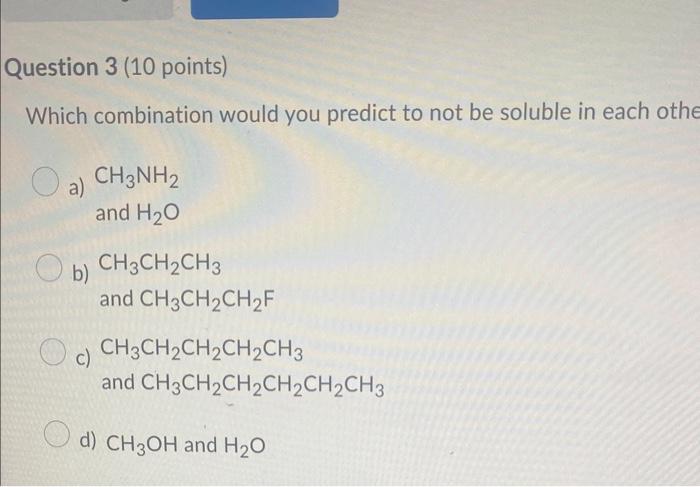
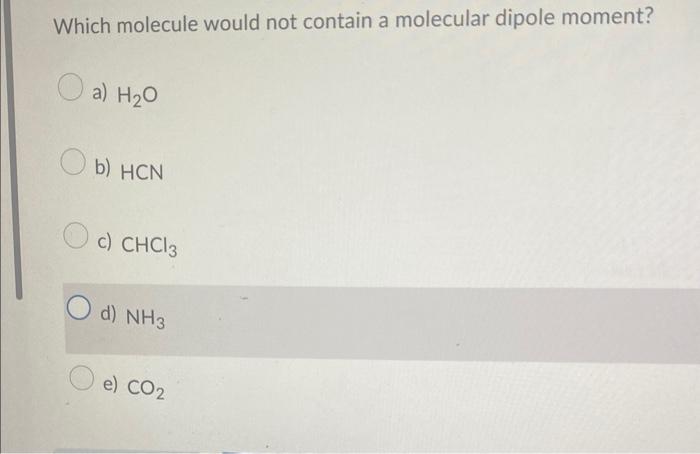
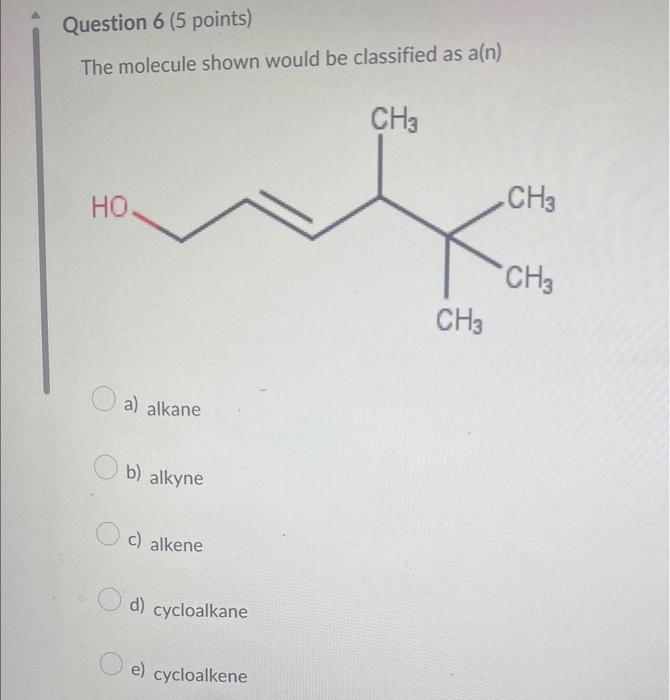


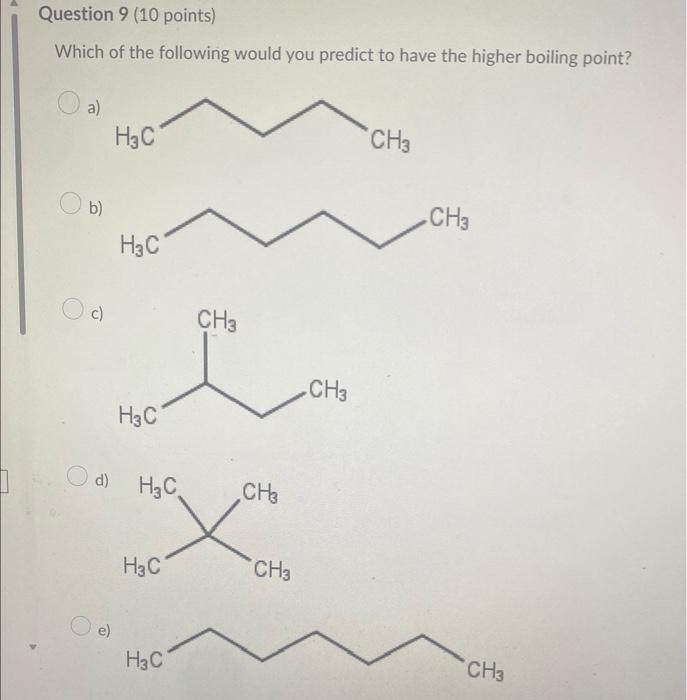
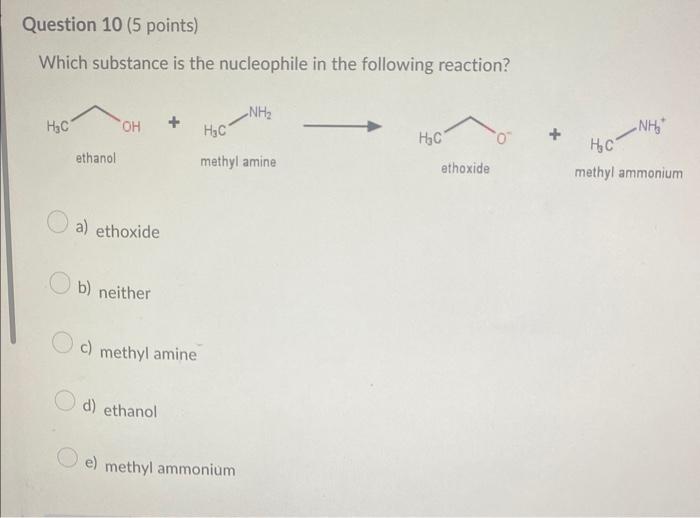
Which compound below represents a hydrocarbon? a) CH3CH2CH2NH2 b) CH3CH=CH2 c) CH3CH2CH2OH d) CH3CH2CH2F Which combination would you predict to not be soluble in each othe a) CH3NH2 and H2O b) CH3CH2CH3 and CH3CH2CH2F c) CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3 d) CH3OH and H2O Which molecule would not contain a molecular dipole moment? a) H2O b) HCN c) CHCl3 d) NH3 e) CO2 The molecule shown would be classified as a(n) a) alkane b) alkyne c) alkene d) cycloalkane e) cycloalkene Which of the following would you predict to be the most soluble in water? a) CH3COCH3 b) CH3CH2OCH3 c) CH3CH2COOH d) CH3CH2CHO Which of the following would be the strongest acid? a) H2O b) H2S c) HBr d) HCl Which of the following would you predict to have the higher boiling point? Which substance is the nucleophile in the following reaction? +H3CNH2 ethanol methyl amine ethoxide methyl ammonium a) ethoxide b) neither c) methyl amine d) ethanol e) methyl ammonium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


