Question: Which compound is more basic and why? I is more basic because of the localized electrons on the nitrogen atom I is more basic because
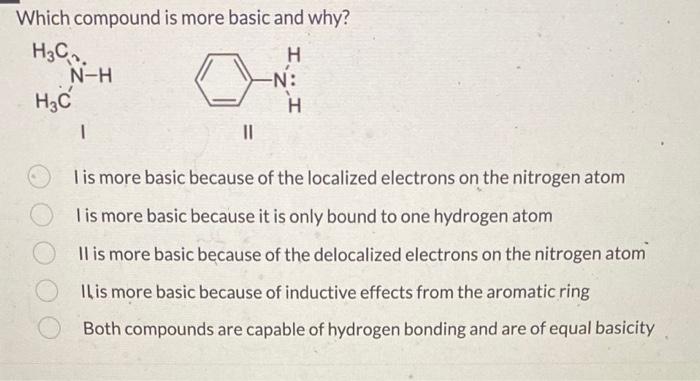
Which compound is more basic and why? I is more basic because of the localized electrons on the nitrogen atom I is more basic because it is only bound to one hydrogen atom II is more basic because of the delocalized electrons on the nitrogen atom IL is more basic because of inductive effects from the aromatic ring Both compounds are capable of hydrogen bonding and are of equal basicity
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


