Question: Which element, indicated by letters on the periodic table, has the electron configuration 1s22s22p63s23p64s2 ? Ar Ca Se K Use the molecular orbital diagram shown
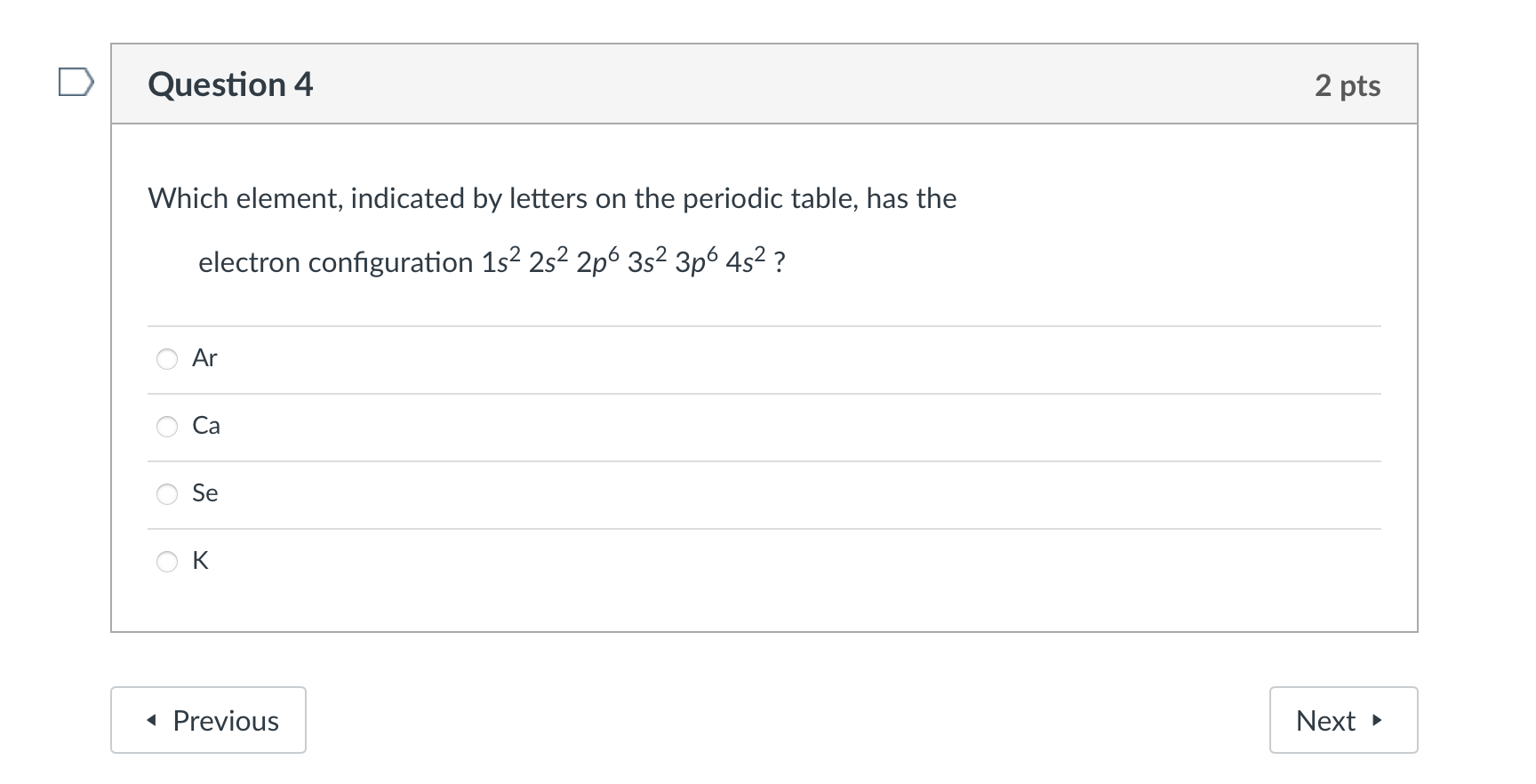
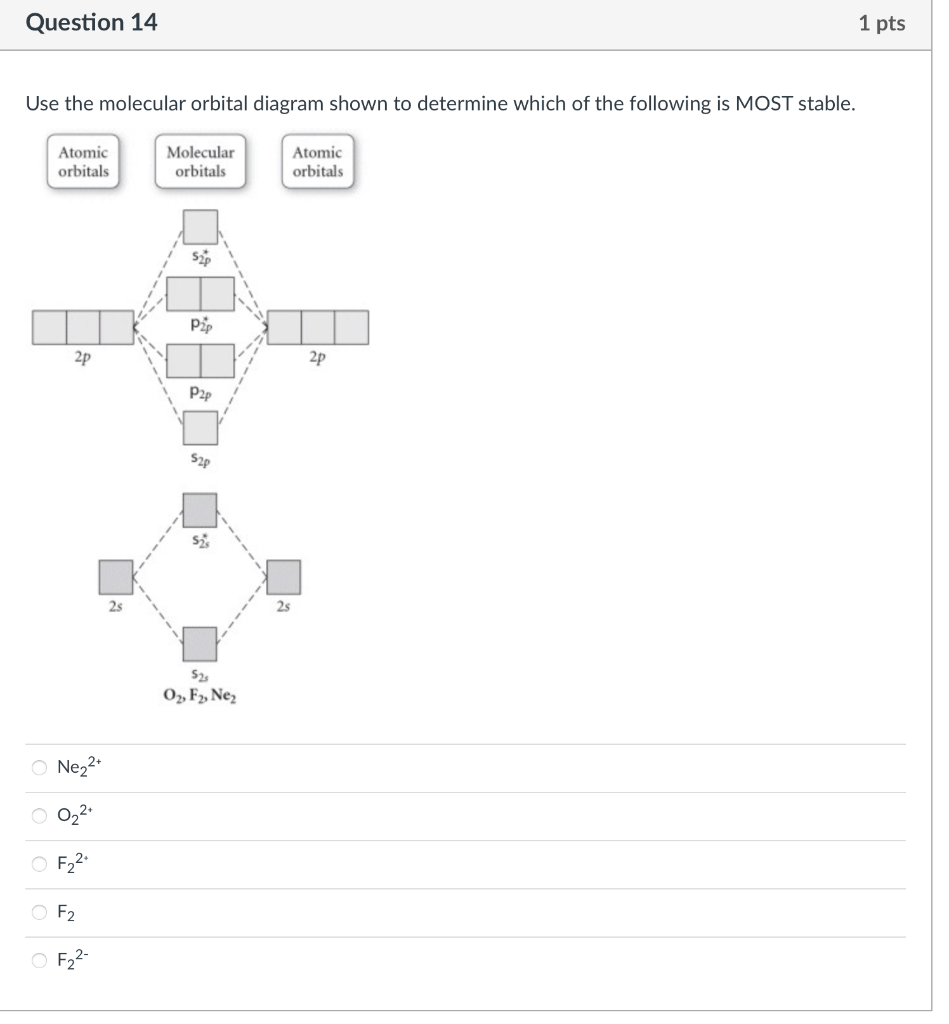
Which element, indicated by letters on the periodic table, has the electron configuration 1s22s22p63s23p64s2 ? Ar Ca Se K Use the molecular orbital diagram shown to determine which of the following is MOST stable. \begin{tabular}{|l|l|} \hline AtomicorbitalsMolecularorbitalsAtomicorbitals \\ \hline \end{tabular} Ne22+O22+F22+F2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


