Question: which molecule has a higher bliling pount. explain based on IMF! they both have 6 Carbons 2. For the molecules below, circle the one you'd
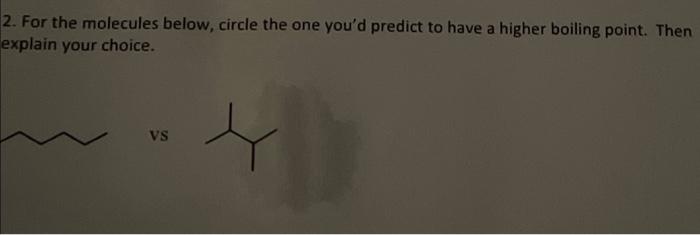
2. For the molecules below, circle the one you'd predict to have a higher boiling point. Then explain your choice. vS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


